Abstract
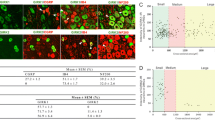
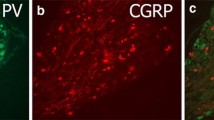
Rat lumbar dorsal root ganglion neurones projecting to the nucleus gracilis in the brainstem were retrogradely labelled with Fluoro-Gold and analysed immunocytochemically for their expression of substance P-, calcitonin gene-related peptide-, galanin-, galanin message-associated peptide-, neuropeptide Y-, nitric oxide synthase- and carbonic anhydrase-like immunoreactivity as well as affinity to Griffonia (bandeiraea) simplicifolia lectin I — isolectin B4, RT97 and to choleragenoid. The analysis was made both in uninjured rats and in rats which had been subjected to unilateral sciatic nerve transection and partial resection 3 weeks earlier. The data showed that 6% of the L4 and L5 lumbar dorsal root ganglion cells that projected to the nucleus gracilis showed substance P-like immunoreactivity. Following nerve injury, none of the nucleus gracilis-projecting dorsal root ganglion cells showed substance P-like immunoreactivity. Nineteen per cent of the investigated cell population showed calcitonin gene-related peptide-like immunoreactivity in uninjured rats, but no nucleus gracilisprojecting calcitonin gene-related peptide-positive cells were found after nerve injury. Galanin- and galanin message-associated peptide-like immunoreactivity were found in 2% and 3%, respectively, of the Fluoro-Gold-labelled cell population normally and in 22% and 14%, respectively, after injury. No neuropeptide Y-positive cells were found in the Fluoro-Gold-labelled cell population normally, but after nerve injury, 96% of this population became neuropeptide Y-positive. Nitric oxide synthaselike immunoreactivity was found in 2% of the Fluoro-Gold-labelled cells normally and in 10% after injury. Two per cent of the Fluoro-Gold-labelled cells in the normal cases were stained by Griffonia (bandeiraea) simplicifolia lectin I — isolectin B4. After injury, however, no such double labelling was found. Thirty-four per cent of the Fluoro-Gold-labelled cell population was carbonic anhydrase positive normally, and 42% after injury. Seventy-five per cent of the Fluoro-Gold-labelled cells showed RT97 immunoreactivity normally and 12% after injury. Choleragenoid-like immunoreactivity was found in 99% of the Fluoro-Gold-labelled dorsal root ganglion cells normally and 81% after injury.
Immunohistochemical visualisation of choleragenoid transganglionically transported from the injured sciatic nerve combined with neuropeptide Y immunocytochemistry showed that primary afferent fibres and terminals in the nucleus gracilis contain neuropeptide Y following peripheral nerve transection. Taken together, the results indicate that peripherally axotomised nucleus gracilis-projecting neurones undergo marked alterations in their cytochemical characteristics, which may be significant for the structural and functional plasticity of this system after injury.
Similar content being viewed by others
References
Aimi Y, Fujimura M, Vincent SR, Kimura H (1991) Localization of NADPH-diaphorase-containing neurons in sensory ganglia of the rat. J Comp Neurol 306:382–392
Albright BC (1989) The morphology of primary afferent terminals in the rat gracile nucleus following peripheral nerve crush injury. Anat Rec 223:7A
Aldskogius H, Arvidsson J, Hansson P (1988) Carbonic anhydrase enzyme histochemistry of cranial nerve primary sensory afferent neurons in the rat. Histochemistry 88:151–154
Aldskogius H, Arvidsson J, Grant G (1992) Axotomy-induced changes in primary sensory neurons. In: Scott SA (ed) Sensory neurons — diversity, development and plasticity. Oxford University Press, New York, pp 363–383
Allen JM, Adrian TE, Tatemoto K, Polak JM, Hughes J, Bloom SR (1982) Two novel related peptides, neuropeptide Y (NPY) and peptide YY (PYY) inhibit the contraction of the electrically stimulated mouse vas deferens. Neuropeptides 3:71–77
Anderton B, Coakham HB, Garson JA, Harper AA, Harper EI, Lawson SN (1983) A monoclonal antibody against neurofilament protein specifically labels the large light cell population in rat dorsal root ganglia. J Physiol (Lond) 334:97P-98P
Andres KH (1961) Untersuchungen über den Feinbau von Spinalganglien. Z Zellforsch 55:1–48
Barbut D, Polak JM, Wall PD (1981) Substance P in spinal cord dorsal horn decreases following peripheral nerve injury. Brain Res 205:289–298
Barron KD, Doolin PF (1969) Neuronal responses to axon injury. In: Norris FH, Kurland LT (eds) Motor neuron diseases. Grune and Stratton, New York, pp 301–318
Barron KD, Tuncbay TO (1962) Phosphatase in cuneate nuclei after brachial plexectomy. Arch Neurol 7:203–210
Bleakman D, Colmers WF, Fournier A, Miller RJ (1991) Neuropeptide Y inhibits Ca2+ influx into cultured dorsal root ganglion neurones of the rat via a Y2 receptor. Br J Pharmacol 103:1781–1789
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347:768–770
Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH (1991) Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7:615–624
Brown AG (1981) Organization in the spinal cord: the anatomy and physiology of identified neurones. Springer, Berlin Heidelberg New York
Bullitt E (1990) Expression of C-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296:517–530
Burgess PR, Clark FJ (1969) Dorsal column projection of fibres from the cat knee joint. J Physiol (Lond) 203:301–315
Ch'ng JLC, Christofides ND, Anand P, Gibson SJ, Allen YS, Su HC, Tatemoto K, Morrison JFB, Polak JM, Bloom SR (1985) Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience 16:343–354
Chung K, Coggeshall RE (1985) Unmyelinated primary afferent fibers in dorsal funiculi of cat sacral spinal cord. J Comp Neurol 238:365–369
Chung K, Langford LA, Coggeshall RE (1987) Primary afferent and propriospinal fibers in the rat dorsal and dorsolateral funiculi. J Comp Neurol 363:68–75
Colmers WF, Klapstein GJ, Fournier A, St-Pierre S, Treherne KA (1991) Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol 102:41–44
Colmers WF, Lukowiak K, Pittmann QJ (1985) Neuropeptide Y reduces orthodromically evoked population spike in rat hippocampal CA1 by a possibly presynaptic mechanism. Brain Res 346:404–408
Conti F, De Biasi S, Giuffrida R, Rustioni A (1990) Substance P-containing projections in the dorsal columns of rats and cats. Neuroscience 34:607–621
Dahlström A (1968) Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol 5:111–113
Dalsgaard CJ (1988) The sensory system. In: Björklund A, Hökfelt T, Owman C (eds) Handbook of chemical neuroanatomy, vol 6. The peripheral nervous system. Elsevier, Amsterdam, pp 599–636
Devor M (1989) The pathophysiology of damaged peripheral nerves. In: Wall PD, Melzack R (eds) Textbook of pain. Churchill Livingstone, Edinburgh, pp 63–81
Devor M, Claman D (1980) Mapping and plasticity of acid phosphatase afferents in the rat dorsal horn. Brain Res 190:17–28
Dräger UC, Hofbauer A (1984) Antibodies to heavy neurofilament subunit detect a subpopulation of damaged ganglion cells in retina. Nature 309:624–626
Duce IR, Keen P (1977) An ultrastructural classification of the neuronal cell bodies of the rat dorsal root ganglion using zinc iodide-osmium impregnation. Cell Tissue Res 185:263–277
Dumoulin FL, Raivich G, Streit WJ, Kreutzberg GW (1991) Differential regulation of calcitonin gene-related peptide (CGRP) in regenerating rat facial nucleus and dorsal root ganglion. Eur J Neurosci 3:338–342
Eriksson NP, Persson JKE, Svensson M, Arvidsson J, Molander C, Aldskogius H (1993a) A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res 96:19–27
Eriksson NP, Persson JKE, Svensson M, Meijer B, Aldskogius H (1993b) A quantitative analysis of astroglial and microglial cell reactions in primary sensory projection areas following sciatic nerve injury in the adult rat. Soc Neurosci Abstr 19:451
Ewald DA, Matthies HJG, Perney TM, Walker MW, Miller RJ (1988) The effect of down regulation of protein kinase C on the inhibitory modulation of dorsal root ganglion neuron Ca2+ currents by neuropeptide Y. J Neurosci 8:2447–2451
Fabri M, Conti F (1990) Calcitonin gene-related peptide-positive neurons and fibers in the cat dorsal column nuclei. Neuroscience 35:167–174
Fiallos-Estrada CE, Kummer W, Mayer B, Bravo R, Zimmermann M, Herdegen T (1993) Long-lasting increase of nitric oxide synthase immunoreactivity, NADPH-diaphorase reaction and c-JUN co-expression in rat dorsal root ganglion neurons following sciatic nerve transection. Neurosci Lett 150:169–173
Fried K, Arvidsson J, Robertson B, Pfaller K (1991) Anterograde horseradish peroxidase tracing and immunohistochemistry of trigeminal ganglion tooth pulp neurons after dental nerve lesions in the rat. Neuroscience 43:269–278
Frisén J, Risling M, Theodorsson E, Fried K (1992) NPY-like immunoreactivity in sensory nerve fibers in rat sciatic neuroma. Brain Res 577:142–146
Gehrmann J, Monaco S, Kreutzberg GW (1991) Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor Neurol Neurosci 2:181–198
Gibson SJ, Polak JM, Allen JM, Adrian TE, Kelly JS, Bloom SR (1984) The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J Comp Neurol 227:78–91
Giesler GJ Jr, Nahin RL, Madsen AM (1984) Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol 51:260–275
Giuliani S, Maggi CA, Meli A (19889) Prejunctional modulatory action of neuropeptide Y on peripheral terminals of capsaicinsensitive sensory nerves. Br J Pharmacol 98:407–412
Goldstein ME, Cooper HS, Bruce J, Carden MJ, Lee VM-Y, Schlaepfer WW (1987) Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve. J Neurosci 7:1586–1594
Goldstein M, Deutch AY (1989) The inhibitory actions of NPY and galanin on 3H-norepinephrine release in the central nervous system: relation to a proposed heirarchy of neuronal coexistence. In: Mutt V, Fuxe K, Hökfelt H, Lundberg JM (eds) Neuropeptide Y. Raven Press, New York, pp 153–162
Gordon G, Paine CH (1960) Functional organization in nucleus gracilis of the cat. J Physiol (Lond) 153:331–349
Grant G, Arvidsson J, Robertson B, Ygge J (1979) Transganglion ic transport of horseradish peroxidase in primary sensory neurons. Neurosci Lett 12:23–28
Haas HL, Hermann A, Greene RW, Chan-Palay V (1987) Action and location of neuropeptide tyrosine (Y) on hyppocampal neurons of the rat in slice preparations. J Comp Neurol 257:208–215
Hachisuka K, Lais AC, Dyck PJ (1989) Ultrastructural alterations of primary afferent axons in the nucleus gracilis after peripheral nerve axotomy. J Neuropathol Exp Neurol 48:413–424
Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal ganglion neurones. J Physiol (Lond) 359:31–46
Hayes CE, Goldstein IJ (1974) An alpha-d-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem 249:1904–1914
Henken DB, Battisti WP, Chesselet MF, Murray M, Tessler A (1990) Expression of β-preprotachykinin mRNA and tachykinins in rat dorsal root ganglion cells following peripheral or central axotomy. Neuroscience 39:733–742
Hope BT, Michael GJ, Knigge KM, Vincent SR (1991) Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA 88:2811–2814
Hunt SP, Pini A, Evan G (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328:632–634
Hökfelt T, Dahlström A (1971) Effects of two mitosis inhibitors (colchicine and vinblastine) on the distribution and axonal transport of noradrenaline storage particles, studied by fluorescence and electron microscopy. Z Zellforsch 119:460–482
Hökfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T (1987) Increase of galanin-like immunoreactivity in rat dorsal ganglion cells after peripheral axotomy. Neurosci Lett 83:217–220
Hökfelt T, Åman K, Arvidsson U, Bedecs K, Ceccatelli S, Hulting A-L, Langel U, Meister B, Pieribone V, Bartfai T (1992) Galanin message-associated peptide (GMAP)- and galanin-like immunoreactivities: overlapping and differential distributions in the rat. Neurosci Lett 142:139–142
Jenkins R, McMahon SB, Bond AB, Hunt SP (1993) Expression of c-jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci 5:751–759
Jessell T, Tsunoo A, Kanazawa I, Otsuka M (1979) Substance P: depletion in the dorsal horn of rat spinal cord after section of the peripheral processes of primary sensory neurons. Brain Res 168:247–259
Ju G, Hökfelt T, Brodin E, Fahrenkrug J, Fischer J, Frey P, Elde R, Brown J (1987) Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res 247:417–431
Kalaska J, Pomeranz B (1982) Chronic peripheral nerve injuries alter the somatotopic organization of the cuneate nucleus in kittens. Brain Res 236:35–47
Kilborn MJ, Potter EK, McCloskey DI (1985) Neuromodulation of the cardiac vagus: comparison of neuropeptide Y and related peptides. Regul Pept 12:155–161
Kreutzberg GW (1969) Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci USA 62:722–728
Lahr SP, Stelzner DJ (1990) Anatomical studies of dorsal column axons and dorsal root ganglion cells after spinal cord injury in the newborn rat. J Comp Neurol 293:377–398
LaMotte CC, Kapadia SE, Shapiro CM (1991) Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoidHRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP). J Comp Neurol 311:546–562
Lawson SN, Harper AA, Garson JA, Anderton BH (1984) A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol 228:263–272
Lee J-H, Wilcox GL, Beitz AJ (1992) Nitric oxide mediates Fos expression in the spinal cord induced by mechanical noxious stimulation. Neuro Report 3:841–844
Leong S-K, Ling E-A (1990) Labelling neurons with fluorescent dyes administered via intravenous, subcutaneous or intraperitoneal route. J Neurosci Methods 32:15–23
Lundberg JM, Terenius L, Hökfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom SR, Goldstein M (1982) Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116:477–480
Lönnerholm G, Midtvedt T, Schenholm M, Wistrand PJ (1988) Carbonic anhydrase isoenzymes in the caecum and colon of normal and germ-free rats. Acta Physiol Scand 132:159–166
McQuarrie IG (1983) Role of the axonal cytoskeleton in the regeneration nervous system. In: Seil FJ (ed) Nerve, organ and tissue regeneration. Academic Press, New York, pp 51–88
Mense S, Craig AD Jr (1988) Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience 26:1023–1035
Molander C, Hongpaisan J, Grant C (1992) Changing pattern of c-fos expression in spinal cord neurons after electrical stimulation of the chronically injured sciatic nerve in the rat. Neuroscience 50:223–236
Moradian GP, Rustioni A (1977) Transganglionic degeneration in the dorsal horn and dorsal column nuclei of adult rats. Anat Rec 187:660 (Abstract)
Moss TH, Lewkowicz SJ (1983) The axon reaction in motor and sensory neurons of mice studied by a monoclonal antibody marker of neurofilament protein. J Neurol Sci 60:267–280
Nagy JI, Hunt SP (1982) Fluoride-resistant acid phosphatase-containing neurons in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience 7:89–97
Nielsch U, Bisby MA, Keen P (1987) Effect of cutting or crushing the rat sciatic nerve on synthesis of substance P by isolated L5 dorsal root ganglia. Neuropeptides 10:137–145
Nielsch U, Keen P (1989) Reciprocal regulation of tachykininand vasoactive intestinal polypeptide-gene expression in rat sensory neurones following cut and crush injury. Brain Res 481:25–30
Noguchi K, Senba E, Morita Y, Sato M, Tohyama M (1989) Prepro-VIP and preprotachykinin mRNAs in the rat dorsal root ganglion cells following peripheral axotomy. Mol Brain Res 6:327–330
Noguchi K, Senba E, Morita Y, Sato M, Tohyama M (1990) α-GCGRP and β-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Mol Brain Res 7:299–304
Noguchi K, Kawai Y, Senba E (1993) DRG neurons projecting to dorsal column nuclei express preprotachykinin mRNA after peripheral axotomy. Soc Neurosci Abstr 19:519
Oblinger MM, Lasek RJ (1988) Axotomy-induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J Neurosci 8:1747–1758
Pannese E (1963) Investigations on the ultrastructural changes in the spinal ganglion neurons in the course of axon regeneration and cell hypertrophy. Z Zellforsch 61:561–586
Patterson JT, Head PA, McNeill DL, Chung K, Coggeshall RE (1989) Ascending unmyelinated primary afferent fibers in the dorsal funiculus. J Comp Neurol 290:384–390
Patterson JT, Coggeshall RE, Lee WT, Chung K (1990) Long ascending unmyelinated primary afferent axons in the rat dorsal column: immunohistochemical localizations. Neurosci Lett 108:6–10
Persson JKE, Aldskogius H, Arvidsson J, Holmberg A (1991) Ultrastructural changes in the gracile nucleus of the rat after sciatic nerve transection. Anat Embryol 184:591–604
Persson JKE, Hongpaisan J, Molander C (1993) C-fos expression in gracilothalamic tract neurons after electrical stimulation of the injured sciatic nerve in the adult rat. Somatosens Mot Res 10:475–483
Peyronnard JM, Charron L, Lavoie J, Messier JP, Dubreuil M (1988a) Carbonic anhydrase and horseradish peroxidase: double labelling of rat dorsal root ganglion neurons innervating motor and sensory peripheral nerves. Anat Embryol 177:353–359
Peyronnard JM, Charron LF, Messier JP, Lavoie J (1988b) Differential effects of distal and proximal nerve lesions on carbonic anhydrase activity in rat primary sensory neurons, ventral and dorsal root axons. Exp Brain Res 70:550–560
Pieribone VA, Aston-Jones J (1988) The iontophoretic application of Fluoro-Gold for the study of afferents of deep brain nuclei. Brain Res 475:259–271
Price DL, Porter KR (1972) The response of ventral horn neurons to axonal transection. J Cell Biol 53:24–37
Rambourg A, Clermont Y, Beaudet A (1983) Ultrastructural features of six types of neurons in rat dorsal root ganglia. J Neurocytol 12:47–66
Riley DA, Ellis S, Bain JLW (1984) Ultrastructural cytochemical localization of carbonic anhydrase activity in rat peripheral sensory and motor nerves, dorsal root ganglia and dorsal column nuclei. Neuroscience 13:189–206
Rivero-Melián C, Arvidsson J (1992) Brain stem projections of rat lumbar dorsal root ganglia studied with choleragenoid conjugated horseradish peroxidase. Exp Brain Res 91:12–20
Robertson B, Grant G (1985) A comparison between wheat germ agglutinin- and choleragenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurones in the rat with some observations in the cat. Neuroscience 14:895–905
Robertson B, Grant G (1989) Immunocytochemical evidence for the localization of the GM1 ganglioside in carbonic anhydrase-containing and RT 97-immunoreactive rat primary sensory neurons. J Neurocytol 18:77–86
Rökaeus Å, Brownstein MJ (1986) Construction of a porcine adrenal medullary cDNA library and nucleotide sequence analysis of two clones encoding a galanin precursor. Proc Natl Acad Sci USA 83:6287–6291
Schmued LC, Fallon JH (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377:147–154
Schreyer DJ, Skene JHP (1991) Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci 11:3738–3751
Shehab SAS, Atkinson ME (1986) Vasoactive intestinal polypeptide increases in areas of the dorsal horn of the spinal cord from which other neuropeptides are depleted following peripheral axotomy. Exp Brain Res 62:422–430
Silverman JD, Kruger L (1988) Lectin and neuropeptide labelling of separate populations of dorsal root ganglion neurons and associated “nociceptor” thin axons in rat testis and cornea whole-mount preparations. Somatosens Res 5:259–267
Sinicropi DV, McIlwain DL (1983) Changes in the amounts of cytoskeletal proteins within the perikarya and axons of regenerating frog motoneurons. J Cell Biol 96:240–247
Skofitsch G, Jacobowitz DM (1985) Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res Bull 15:191–195
Sommer EW, Kazimierczak J, Droz B (1985) Neuronal subpopulations in the dorsal root ganglion of the mouse as characterized by combination of ultrastructural and cytochemical features. Brain Res 346:310–326
Streit WJ, Schulte BA, Balentine JD, Spicer SS (1985) Histochemical localization of galactose-containing glycoconjugates in sensory neurons and their processes in the central and peripheral nervous system of the rat. J Histochem Cytochem 33:1042–1052
Sugimoto T, Bennett GJ, Kajander KC (1990) Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain 42:205–213
Tamatani M, Senba E, Tohyama M (1989) Calcitonin gene-related peptide- and substance P-containing primary afferent fibers in the dorsal column of the rat. Brain Res 495:122–130
Tenser RB (1985) Sequential changes of sensory neuron (fluoride-resistant) acid phosphatase in dorsal root ganglion neurons following neurectomy and rhizotomy. Brain Res 332:386–389
Torvik A (1976) Central chromatolysis and the axon reaction: a reappraisal. Neuropathol Appl Neurobiol 2:423–432
Valtschanoff JG, Weinberg RJ, Rustioni A (1993) Nitric oxide synthase and amino acid neurotransmitters in the rat dorsal column nuclei. Soc Neurosci Abstr 19:328
Verge VMK, Xu Z, Xu X-J, Wiesenfeld-Hallin Z, Hökfelt T (1992) Marked increase in nitric oxide synthase mRNA in rat dorsal root ganglia after peripheral axotomy: in situ hybridization and functional studies. Proc Natl Acad Sci USA 89:11617–11621
Verge VMK, Wiesenfeld-Hallin Z, Hökfelt T (1993) Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey. Eur J Neurosci 5:240–250
Villar MJ, Cortés R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hökfelt T (1989) Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience 33:587–604
Wakisaka S, Kajander KC, Bennett GJ (1991) Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett 124:200–203
Wakisaka S, Kajander KC, Bennett GJ (1992) Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Res 598:349–352
Walker MW, Ewald DA, Perney TM, Miller RJ (1988) Neuropeptide Y modulates neurotransmitter release and Ca2+ currents in rat sensory neurons. J Neurosci 8:2438–2446
Wang H, Rivero-Melián C, Robertson B, Grant G (1994) Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience 62:539–551
Whitsel BL, Petrucelli LM, Sapiro G (1969) Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res 15:67–78
Wiesenfeld-Hallin Z, Hökfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA (1984) Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett 52:199–204
Willis WD Jr, Coggeshall RE (1991) Sensory mechanisms of the spinal cord. Plenum Press, New York
Wong J, Oblinger MM (1987) Changes in neurofilament gene expression occur after axotomy of dorsal root ganglion neurons: an in situ hybridization study. Metab Brain Dis 2:291–303
Wong V, Barrett CP, Donati EJ, Eng LF, Guth L (1983) Carbonic anhydrase activity in first-order sensory neurons of the rat. J Histochem Cytochem 31:293–300
Wood JN, Anderton BH (1981) Monoclonal antibodies to mammalian neurofilaments. Biosci Rep 1:263–268
Woolf CJ (1987) Central terminations of cutaneous mechanoreceptive afferents in the rat lumbar spinal cord. J Comp Neurol 261:105–119
Woolf CJ, Reynolds ML, Molander C, O'Brien C, Lindsay RM, Benowitz LI (1990) The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury. Neuroscience 34:465–478
Yamadori T (1970) A light and electron microscopic study on the postnatal development of spinal ganglia in rats. Acta Anat Nippon 45:191–2005
Yoshikawa H, Tarui S, Hashimoto PH (1985) Diminished retrograde transport causes axonal dystrophy in the nucleus gracilis. Electron- and light-microscopic study. Acta Neuropathol 68:93–100
Zhang X (1994) Messenger plasticity in primary, sensory neurons following peripheral nerve injury. Karolinska Institutet. Thesis
Zhang X, Meister B, Elde R, Verge VMK, Hökfelt T (1993a) Large calibre primary afferent neurons projecting to the gracile nucleus express neuropeptide Y after sciatic nerve lesions: an immunohistochemical and in situ hybridization study in rats. Eur J Neurosci 5:1510–1519
Zhang X, Verge V, Wiesenfeld-Hallin Z, Ju G, Bredt D, Snyder SH, Hökfelt T (1993b) Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol 335:563–575
Zhang X, Wiesenfeld-Hallin Z, Hökfelt T (1994) Effect of peripheral axotomy on expression of neuropeptide Y receptor mRNA in rat lumbar dorsal root ganglia. Eur J Neurosci 6:43–57
Author information
Authors and Affiliations
Additional information
Björn Lindh, MD, PhD, one of the promotors of this study, unexpectedly died on 29 July 1991, shortly after the start of the experimental analysis. His dead is a great loss for the collaborating authors personally and for neuroscience.
Rights and permissions
About this article
Cite this article
Persson, J.K.E., Lindh, B., Elde, R. et al. The expression of different cytochemical markers in normal and axotomised dorsal root ganglion cells projecting to the nucleus gracilis in the adult rat. Exp Brain Res 105, 331–344 (1990). https://doi.org/10.1007/BF00233034
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00233034