Abstract
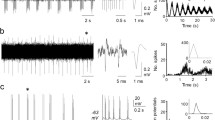
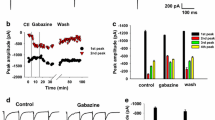
An extracellular microstimulation technique has been used to investigate and compare the properties of group I primary afferent myelinated fibres in the dorsal column and group Ia unmyelinated terminations in the lumbar spinal cord of cats anaesthetised with pentobarbitone sodium. Fibres were distinguished from terminations on the basis of location, anodic blocking factor and sensitivity to GABAA mimetics. The recovery curves of threshold following an orthodromic impulse provided an estimate of both action potential duration and rate of repolarization. The action potentials of group Ia terminations were of briefer duration (by a factor of approximately 2) with more rapid rates of repolarization (factor of approximately 3) than those of the myelinated fibres. The prolongation of termination but not fibre action potentials by microelectrophoretic tetraethylammonium and 4-aminopyridine indicated the presence of voltage-activated potassium channels in the termination membrane. Differences in the effects on Ia termination action potentials of depolarizations (reductions in threshold) associated with a preceding action potential, synaptically released GABA, microelectrophoretic piperidine-4-sulphonic acid or dl-homocysteic acid suggest that an increase in termination membrane conductance is the major factor in the reduction of transmitter release during the activation of presynaptic GABAA receptors.
Similar content being viewed by others
References
Allen C, Stevens CF (1994) An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci 91:10380–10383
Augustine GJ, Charlton MP, Smith SJ (1987) Calcium action in synaptic transmitter release. Annu Rev Neurosci 10:633–693
Barrett EF, Barrett JN (1982) Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol (Lond) 323:117–144
Blight AR, Someya S (1985) Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience 15:1–12
Brigant JL, Mallart A (1982) Presynaptic currents in mouse motor endings. J Physiol (Lond) 333:619–636
Clements JD, Forsythe ID, Redman SJ (1987) Presynaptic inhibition of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia axons. J Physiol (Lond) 383:152–169
Conradi S (1969) Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand [Suppl] 332:5–48
Conradi S, Cullheim S, Gollvik L, Kellerth J-O (1983) Electron microscopic observations on the synaptic contacts of group Ia muscle spindle afferents in the cat lumbosacral spinal cord. Brain Res 265:31–39
Creed RS, Denny-Brown D, Eccles JC, Liddell EGT, Sherrington CS (1932) Reflex activity of the spinal cord. Oxford University Press, Oxford
Curtis DR (1979) A method for continuously monitoring the electrical threshold of single intraspinal nerve fibres. Electroencephalogr Neurophysiol 47:503–506
Curtis DR, Lacey G (1994) Gaba-B receptor-mediated spinal inhibition. Neuroreport 5:540–542
Curtis DR, Lodge D (1982) The depolarization of feline ventral horn group Ia spinal afferent terminations by GABA. Exp Brain Res 46:215–233
Curtis DR, Lodge D, Bornstein JC, Peet MJ, Leah JD (1982) The dual effects of GABA and related amino acids on the electrical threshold of ventral horn group Ia afferent terminations in the cat. Exp Brain Res 48:387–400
Curtis DR, Headley PM, Lodge D (1984a) Depolarization of feline primary afferent fibres by acidic amino acids. J Physiol (Lond) 351:461–472
Curtis DR, Malik R, Leah JD (1984b) The effects of naloxone, morphine and methionine enkephalinamide on Ia afferent terminations in the cat spinal cord. Brain Res 303:289–298
David G, Barrett JN, Barrett EF (1993) Activation of internodal potassium conductance in rat myelinated axons. J Physiol (Lond) 472:177–202
Del Castillo J, Katz B (1956) Localization of active spots within the neuro-muscular junction of the frog. J Physiol (Lond) 132:630–649
Eccles JC (1957) The physiology of nerve cells. Johns Hopkins University Press, Baltimore
Eccles JC, Krnjevic K (1959a) Potential changes recorded inside primary afferent fibres within the spinal cord. J Physiol (Lond) 149:250–273
Eccles JC, Krnjevic K (1959b) Presynaptic changes associated with post-tetanic potentiation in the spinal cord. J Physiol (Lond) 149:274–287
Eccles JC, Kozac W, Magni F (1961) Dorsal root reflexes of muscle group I afferent fibres. J Physiol (Lond) 159:128–146
Eccles JC, Magni F, Willis WD (1962) Depolarization of central terminals of group I afferent fibres from muscle. J Physiol (Lond) 160:62–93
Eccles JC, Schmidt RF, Willis WD (1963) The mode of operation of the synaptic mechanism producing presynaptic inhibition. J Neurophysiol 26:523–538
Fyffe REW (1979) The morphology of group II muscle afferent fibre collaterals (abstract). J Physiol (Lond) 296:39–40P
Fyffe REW, Light AR (1984) The ultrastructure of group Ia afferent fibre synapses in the lumbosacral spinal cord of the cat. Brain Res 300:201–209
Galvan M, Franz P, Vogel-Wiens C (1984) Actions of potassium channel blockers on guinea-pig lateral olfactory tract axons. Arch Pharm 325:8–11
Goldstein SS, Rall W (1974) Changes in action potential shape and velocity for changing core conductor geometry. Biophys J 14:731–753
Graham B, Redman S (1994) A simulation of APs in synaptic boutons during presynaptic inhibition. J Neurophysiol 71:538–549
Gynther BD, Curtis DR (1989) Post-tetanic influences on primary afferent depolarization in the cat spinal cord. Exp Brain Res 74:365–374
Haas HL, Wieser HG, Yasargil MG (1983) 4-Aminopyridine and fibre potentials in rat and human hippocampal slices. Experientia 39:114–115
Hagiwara S, Tasaki I (1958) A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol (Lond) 143:114–137
Hille B (1992) Ionic channels of excitable membranes. Sinauer Associates, Sunderland, Mass
Holstege JC, Calkoen F (1990) The distribution of GABA in lumbar motoneuronal cell groups. A quantitative ultrastructural study in rat. Brain Res 530:130–137
Hubbard JI, Schmidt RF (1963) An electrophysiological investigation of mammalian motor nerve terminals. J Physiol (Lond) 166:145–167
Jack JJB (1978) Some methods for selective activation of muscle afferent fibres. In: Porter R (ed) Studies in neurophysiology. Cambridge University Press, Cambridge, pp 155–176
Jahr CE, Yoshioka K (1986) IA afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonised by kynurenate. J Physiol (Lond) 370:515–530
Jankowska E, McCrea D, Rudomin P, Sykova E (1981) Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol 46:506–516
Jankowska E, Lundberg A, Rudomin P, Sykova E (1982) Effects of 4-aminopyridine on synaptic transmission in the cat spinal cord. Brain Res 240:117–129
Katz B, Miledi R (1965) Propagation of electric activity in motor nerve terminals. Proc R Soc Lond [Biol] 161:453–482
Katz B, Miledi R (1969) Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol (Lond) 203:459–487
Kirkwood PA, Sears TA (1974) Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature 252:243–244
Kocsis JD, Van der Maelen CP (1979) A supernormal period in central axons following single cell stimulation. Exp Brain Res 36:381–386
Kolb H-A (1990) Potassium channels in excitable and non-excitable cells. Rev Physiol Biochem Pharmacol 115:51–91
Konishi T, Sears TA (1984) Electrical activity of mouse motor nerve terminals. Proc R Soc Lond [Biol] 222:115–120
Krogsgaard-Larsen P, Falch E, Schousboe A, Curtis DR, Lodge D (1980) Piperidine-4-sulphonic acid, a new specific GABA agonist. J Neurochem 34:756–759
Laerum H, Storm JF (1994) Hippocampal long-term potentiation is not accompanied by presynaptic spike broadening, unlike synaptic potentiation by K+ channel blockers. Brain Res 637:349–355
Liske S, Morris ME (1994) Extrasynaptic effects of GABA (γaminobutyric acid) agonists on myelinated axons of perpheral nerve. Can J Physiol Pharmacol 72:368–374
Llinas R, Steinberg IZ, Walton K (1981) Presynaptic calcium currents in squid giant synapse. Biophys J 33:289–321
Luscher H-R, Shiner JS (1990) Computation of action potential propagation and presynaptic bouton activation in terminal arborizations of different geometries. Biophys J 58:1377–1388
Maxwell DJ, Christie WM, Ottersen OP, Storm-Mathisen J (1990a) Terminals of group Ia primary afferent fibres in Clarke's column are enriched with l-glutamate-like immunoreactivity. Brain Res 510:346–350
Maxwell DJ, Christie WM, Short AD, Brown AG (1990b) Direct observations of synapses between GABA-immunoreactive boutons and muscle afferent terminals in lamina VI of the cat's spinal cord. Brain Res 530:215–222
McLaughlin BJ, Barber R, Saito K, Roberts E, Wu JY (1975) Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J Comp Neurol 164:305–322
Pierce JP, Mendell LM (1993) Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci 13:4748–4763
Redman S (1979) Junctional mechanisms at group Ia synapses. Prog Neurobiol 12:33–83
Redman SJ (1990) Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev 70:165–198
Riddell JS, Jankowska E, Huber J (1995) Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. J Physiol (Lond) 483:443–460
Sakatani K, Hassan AZ, Chesler M (1991) GABA-sensitivity of dorsal column axons: an in vitro comparison between adult and neonatal rat spinal cords. Dev Brain Res 61:139–142
Sastry BR (1979a) Calcium and action potentials in primary afferent terminals. Life Sci 24:2193–2200
Sastry BR (1979b) γ-Aminobutyric acid and primary afferent depolarization in feline spinal cord. Can J Physiol Pharmacol 57:1157–1167
Scholfield CN (1988) Presynaptic Na/Ca action potentials in unmyelinated axons of olfactory cortex. Emol J Physiol 411:180–187
Segev I (1990) Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol 63:987–998
Shupliakov O, Ornung G, Brodin L, Uljhake B, Ottersen OP, Storm-Mathisen J, Cullheim S (1993) Immunocytochemical localization of amino acid neurotransmitter candidates in the ventral horn of the cat spinal cord: a light microscopic study. Exp Brain Res 96:404–418
Stanley EF (1993) Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron 11:1007–1011
Sypert GW, Munson JB, Fleshman JW (1980) Effect of presynaptic inhibition on axonal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol 44:792–803
Tkacs NC, Wurster RD (1990) Potassium channel blockade differentially affects the relative refactory period of frog afferent terminals and axons. Cell Molec Neurobiol 10:405–421
Tkacs NC, Wurster RD (1991) Strength-duration and activity-dependent excitability properties of frog afferent axons and their intraspinal projections. J Neurophysiol 65:468–476
Wall PD (1958) Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol (Lond) 142:1–21
Walmsley B, Bolton PS (1994) An in vivo pharmacological study of single group Ia fibre contacts with motoneurones in the cat spinal cord. J Physiol (Lond) 481:731–741
Waxman SG, Ritchie JM (1993) Molecular dissection of the myelinated axon. Ann Neurol 33:121–136
Zhang SJ, Jackson MB (1993) GABA-activated chloride channels in secretory nerve endings. Science 259:531–534
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Curtis, D.R., Gynther, B.D., Beattie, D.T. et al. An in vivo electrophysiological investigation of group Ia afferent fibres and ventral horn terminations in the cat spinal cord. Exp Brain Res 106, 403–417 (1995). https://doi.org/10.1007/BF00231063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231063