Summary
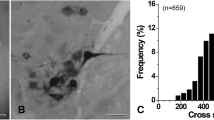
An ultrastructural study of the hypoglossal nucleus (XII) in the rat has revealed two distinct neuronal populations. Hypoglossal motoneurons comprised the largest population of neurons in XII and were identified following injection of horseradish (HRP) into the tongue. Motoneurons were large (25–50 μ), multipolar in shape and distributed throughout XII. The nucleus was large, round and centrally located, and the cytoplasm was characterized by dense lamellar arrays of rough endoplasmic reticulum. In contrast, a second population of small (10–18 μ), round to oval shaped neurons was found restricted to the ventral and dorsolateral regions of XII. The nucleus was markedly invaginated and eccentric, the cytoplasm scant and filled with free ribosomes, and the absence of lamellar arrays of rough endoplasmic reticulum was conspicuous. Neurons of this type were never found to contain HRP reaction product. These results demonstrate that the hypoglossal nucleus does not consist solely of motoneurons, but includes a distinctly separate, presumably non-motoneuronal pool. Arguments are presented in favor of this second neuron population being interneurons. The functional significance of these findings in relation to tongue control is discussed.
Similar content being viewed by others
References
Aldes LD, Boone TB (1984) A combined flat-embedding, HRP histochemical method for correlative light and electron microscopic study of single neurons (in press)
Alley KE (1973) Neuronal morphology and synaptic patterns in the hypoglossal nucleus. J Dent Res 52: 100
Bak IJ, Choi WB (1974) Electron microscopic investigation of synaptic organization of the trochlear nucleus in the cat. I. Normal ultrastructure. Cell Tiss Res 150: 409–423
Barnard JW (1940) The hypoglossal complex of vertebrates. J Comp Neurol 72: 489–524
Conradi S (1976) Functional anatomy of the anterior horn motor neuron. In: Landon DN (ed) The Peripheral Nerve. Wiley & Sons New York, p 291
Cooper MN (1981a) Neurons of the hypoglossal nucleus of the rat. Otolaryngol Head Neck Surg 89: 10–15
Cooper MN (1981b) The hypoglossal nucleus of the primate: A golgi study. Neurosci Lett 21: 249–254
Destombes J, Gogan P, Rouvière A (1979) The fine structure of neurones and cellular relationships in the abducens nucleus in the cat. Exp Brain Res 35: 249–267
Falls WM, King JS (1976) The facial motor nucleus of the opposum: Cytology and axosomatic synapses. J Comp Neurol 167: 177–204
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14: 291–302
Green JD, Negishi K (1963) Membrane potentials in hypoglossal motoneurons. J Neurophysiol 26: 835–856
Hamos JE, King JS (1980) The synaptic organization of the motor nucleus of the trigeminal nerve in the opposum. J Comp Neurol 194: 441–463
Hashimoto PH, Palay SL (1965) Electron microscopy of the dorsal nucleus of the vagus, the hypoglossal nucleus, and the intermediolateral horn of the thoracic cord in rats and cats. Anat Rec 151: 360
Hayashimoto I (1960) Comparative anatomical studies of the hypoglossal nucleus of the carnivore. Bull Kobe Med Coll 20: 394–408
Lodge D, Duggan AW, Biscoe TJ, Cadely KW (1973) Concerning recurrent collaterals and afferent fibers in the hypoglossal nucleus of the rat. Exp Neurol 41: 63–75
Lowe AA (1978) Excitatory and inhibitory inputs to hypoglossal motoneurons and adjacent reticular formation neurons in cats. Exp Neurol 62: 30–47
McLaughlin BJ (1972) The fine structure of neurons and synapses in the motor nuclei of the cat spinal cord. J Comp Neurol 144: 429–460
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106
Morimoto T, Kato, I, Kawamura Y (1966) Studies on functional organization of the hypoglossal nucleus. J Osaka Dent Univ 6: 75–87
Odutola AB (1976) Cell grouping and golgi architecture of the hypoglossal nucleus of the rat. Exp Neurol 52: 356–371
Porter R (1965) Synaptic potentials in hypoglossal motoneurones. J Physiol (Lond) 180: 209–224
Rafols JA, Fox CA (1976) The neurons in the primate subthalamic nucleus: A golgi and electron microscopic study. J Comp Neurol 168: 75–112
Sumi T (1969) Functional differentiation of hypoglossal neurons in the cat. Jpn J Physiol 19: 55–67
Tredici G, Pizzini G, Milanesi S (1976) The ultrastructure of the oculomotor nerve (somatic efferent portion) of the cat. Anat Embryol 149: 323–346
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boone, T.B., Aldes, L.D. The ultrastructure of two distinct neuron populations in the hypoglossal nucleus of the rat. Exp Brain Res 54, 321–326 (1984). https://doi.org/10.1007/BF00236233
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00236233