Summary
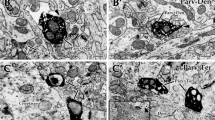
The perigeniculate nucleus of the cat (PGN) was examined at light and electron microscopic levels after immunocytochemical labeling for the gamma-aminobutyric acid (GABA) synthesizing enzyme, glutamic acid decarboxylase (GAD). In light microscopic sections, virtually all perikarya were found to be labeled (GAD+), as well as proximal dendrites, fibres and punctiform elements. Cells in the thalamic reticular nucleus (TRN) dorsal to PGN were also labeled. Ultrastructural analysis of PGN showed immunoreactivity in all somata, in dendrites and in the following vesicle containing profiles: 1.) F1 terminals, which are characterized by large size, dark mitochondria, and pleomorphic vesicles. These terminals form symmetrical synaptic contacts with somata, somatic spines and with dendrites of GAD+ PGN cells. 2.) F2 terminals, which are smaller than F1 terminals, contain also pleomorphic vesicles and frequently make serial synapses of the symmetric type with other F2 terminals. Presumably, F1 terminals are formed by collaterals of PGN-cell axons and F2 terminals by vesicle containing dendrites of PGN cells. Terminals devoid of immunoreactivity included: 1.) RLD terminals characterized by large size, round vesicles, dark mitochondria, and by asymmetric synaptic contacts with somata, especially with somatic spines, and with dendrites of GAD+ perigeniculate neurons; 2.) RSD terminals, characterized by small size, round vesicles and dark mitochondria, which make asymmetric synapses with GAD+ dendrites of medium and small size; 3.) Multivesicular (MV) terminals with variably shaped vesicles including dense core vesicles synapsing on GAD+ dendrites. There are reasons to believe that RSD terminals belong to corticofugal axons and RLD terminals to collateral axons of LGN relay cells. The origin of MV terminals remains to be determined. The GABAergic nature of the PGN cells conforms with the presumed function of these cells as mediators of inhibition of LGN relay cells. The complex synaptic relations observed between GAD+ elements in the PGN would allow for reciprocal inhibition between perigeniculate cells.
Similar content being viewed by others
References
Ahlsen G, Lindström S (1978) Axonal branching of functionally identified neurones in the lateral geniculate body of the cat. Neurosci Lett Suppl 1: 156
Ahlsen G, Lindström S (1982a) Excitation of perigeniculate neurones via axon collaterals of principal cells. Brain Res 236: 477–481
Ahlsen G, Lindström S (1982b) Mutual inhibition between perigeniculate neurones. Brain Res 236: 482–486
Ahlsen G, Lindström S, Sybirska E (1978) Subcortical axon collaterals of principal cells in the lateral geniculate body of the cat. Brain Res 156: 106–109
Ahlsen G, Lindström S, Lo F-S (1980) Projection of brainstem neurones to the perigeniculate nucleus in the cat. Neurosci Lett Suppl 5: 292
Ahlsen G, Grant K, Lindström S (1982a) Monosynaptic excitation of principal cells in the lateral geniculate nucleus by cortifugal fibers. Brain Res 234: 452–458
Ahlsen G, Lindström S, Lo F-S (1982b) Functional distinction of perigeniculate and thalamic reticular neurons in the cat. Exp Brain Res 46: 118–126
Barber RP, Saito K (1976) Light microscopic visualization of GAD and GABA-T in immunocytochemical preparations of rodent CNS. In: Roberts E, Chase TN, Tower DB (eds) Nervous System Function. Raven Press, New York, pp 113–132
Berman AL, Jones EG (1982) The thalamus and basal telencephalon of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. The University of Wisconsin Press, Madison, Wisconsin
Burke W, Cole AM (1978) Extraretinal influences on the lateral geniculate nucleus. Rev Physiol Biochem Pharmacol 80: 105–166
Curtis DR, Tebecis AK (1972) Bicuculline and thalamic inhibition. Exp Brain Res 16: 210–218
Dubin MW, Cleland BG (1977) The organization of visual inputs to interneurons of the lateral geniculate nucleus of the cat. J Neurophysiol 40: 410–427
Famiglietti Jr. EV, Peters A (1972) The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 144: 285–334
Ferster D, LeVay S (1978) The axonal arborization of lateral geniculate neurons in the striate cortex of the cat. J Comp Neurol 182: 923–944
Friedlander MJ, Lin CS, Sherman SM (1979) Structure of physiologically identified X and Y cells in the cats' lateral geniculate nucleus. Science 204: 1114–1117
Friedlander MJ, Lin CS, Stanford LR, Sherman M (1981) Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46: 80–129
Guillery RW (1969) The organization of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch 96: 1–38
Hale PT, Jervie-Sefton A, Baur L, Cottee LJ (1982) Interrelations of the rats thalamic reticular and dorsal lateral geniculate nuclei. Exp Brain Res 45: 217–229
Hendrickson AE, Hunt SP, Wu JY (1981) Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature 292: 605–507
Hendrickson AE, Ogren MP, Vaughn JE, Barber R, Wu JY (1983) Light and electron microscopic immunocytochemical localization of glutamic acid decarboxylase in monkey geniculate complex: Evidence for gabaergic neurons and synapses. J Neurosci 3: 1245–1266
Houser CR, Vaughn JE, Barber R, Roberts E (1980) GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res 200: 341–354
Houser CR, Hendry SHC, Jones EG, Vaughn JE (1983) Morphological diversity of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol 12: 617–638
Ide LS (1982a) The fine structure of the perigeniculate nucleus in the cat. J Comp Neurol 210: 317–334
Ide LS (1982b) Fine structure of the thalamic reticular nucleus in the cat. Soc Neurosci Abstr 8: 261
Laties AM, Sprague JM (1966) The projection of optic fibers to visual centers in the cat. J Comp Neurol 127: 35–70
Lieberman AR (1973) Neurons with presynaptic perikarya and presynaptic dendrites in the rat lateral geniculate nucleus. Brain Res 59: 35–59
Lindström S (1982) Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res 234: 447–453
McLaughlin BJ, Wood JG, Saito K, Barber R, Vaughn JE, Roberts E, Wu JY (1974) The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res 76: 377–391
Montero VM (1983) Ultrastructural identification of axon terminals from the thalamic reticular nucleus in the medial geniculate body in the rat: An EM autoradiographic study. Exp Brain Res 51: 338–342
Montero VM, Scott GL (1981) Synaptic terminals in dorsal lateral geniculate nucleus from neurons of the thalamic reticular nucleus. A light and electron microscope autoradiographic study. Neuroscience 6: 2561–2577
Montero VM, Guillery RW, Woolsey CN (1977) Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res 138: 407–421
Morgan R, Sillito AM, Wolstencroft JH (1974) A pharmacological investigation of inhibition in the lateral geniculate nucleus. J Physiol (Lond) 246: 93–94
Oertel WH, Schmechel DE, Mugnaini E, Tappaz ML, Kopin IJ (1981) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience 6: 2715–2735
Ohara PT, Liberman AR (1981) Thalamic reticular nucleus: anatomical evidence that cortico-reticular axons establish monosynaptic contact with reticulo-geniculate projection cells. Brain Rest 207: 153–156
Ohara PT, Lieberman AR, Hunt SP, Wu JY (1983) Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; Immunohistochemical studies by light and electron microscopy. Neuroscience 8: 189–211
Ribak CE, Vaughn JE, Barber R (1981) Immunocytochemical localization of GABAergic neurons at the electron microscopic level. Histochem J 13: 555–582
Robson JA (1983) The morphology of corticofugal axons to the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 216: 89–103
Saito K, Wu JY, Matsuda T, Roberts E (1974) Immunocytochemical comparisons of vertebrate glutamic acid decarboxylase. Brain Res 65: 277–285
Sanderson KJ (1971) The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol 143: 101–118
Sanderson KJ (1974) Lamination of the dorsal lateral geniculate nucleus in carnivores of the weasel (Mustelidae), raccoon (Procyonidae) and fox (Canidae) families. J Comp Neurol 153: 239–266
Schmielau F (1979) Integration of visual and nonvisual information in nucleus reticularis thalami of the cat. In: Freeman RD (ed) Developmental Neurobiology of Vision. Plenum Press, New York, pp 205–266
Schmielau F, Singer W (1977) The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res 120: 354–361
Singer W (1973) The effect of mesencephalic reticular stimulation on intracellular potentials of cat lateral geniculate neurons. Brain Res 61: 35–54
Singer W (1977) Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev 57: 386–420
So YT, Shapley R (1981) Spatial tuning of cells in and around lateral geniculate nucleus of the cat: X and Y relay cells and perigeniculate interneurons. J Neurophysiol 45: 107–119
Somogyi P, Freund TF, Wu JY, Smith AD (1983) The section-Golgi impregnation procedure. 2. Immunocytochemical demonstration of glutamate decarboxylase in Golgi-impregnated neurons and in their afferent synaptic boutons in the visual cortex of the cat. Neuroscience 10: 261–294
Sotelo C, Palay SL (1971) Altered axons and axon terminals in the lateral vestibular nucleus of the rat. Possible example of axonal remodeling. Lab Invest 25: 653–671
Szentágothai J (1972) Lateral geniculate body structure and eye movement. Bibl Ophthalmol 82: 178–188
Updyke BV (1975) The patterns of projection of cortical area 17, 18 and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 163: 377–396
Updyke BV (1977) Topographic organization of the projections from cortical areas 17, 18 and 19 onto the thalamus, pretectum, and superior colliculus in the cat. J Comp Neurol 173: 81–122
Wood JG, McLaughlin BJ, Vaughn JE (1976) Immunocytochemical localization of GAD in electron microscopic preparations in rodent C.N.S. In: Roberts E, Chase TN, Tower DB (eds) GABA in Nervous System Function. Raven Press, New York, pp 133–148
Wu JY, Matsuda T, Roberts E (1973) Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem 248: 3029–3034
Wu JY, Lin C-T, Brandon C, Chan T-S, Mohler H, Richards JG (1982) Regulation and immunocytochemical characterization of glutamic acid decarboxylase. In: Chan-Palay V, Palay SL (eds) Cytochemical Methods in Neuroanatomy. Alan R. Liss, Inc., New York, pp 279–296
Author information
Authors and Affiliations
Additional information
Supported in part by NIH grants EY02877 to V.M. Montero and HD 03352 to the Waisman Center
Rights and permissions
About this article
Cite this article
Montero, V.M., Singer, W. Ultrastructure and synaptic relations of neural elements containing glutamic acid decarboxylase (GAD) in the perigeniculate nucleus of the cat. Exp Brain Res 56, 115–125 (1984). https://doi.org/10.1007/BF00237447
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237447