Summary
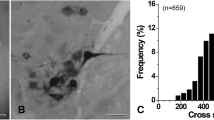
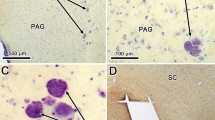
The organization of projections from the principal sensory trigeminal nucleus (PSN) to the hypoglossal nucleus (XII) in the rat was investigated at the light and electron microscopic level with retrograde and anterograde axonal tracer techniques. Microiontophoretic injection of horseradish peroxidase (HRP) into XII resulted in retrograde labeling of neurons confined to the dorsal one-third of the PSN. Labeled neurons were found bilaterally, although a clear preponderance for ipsilateral distribution was evident. Most labeled neurons were found in the medial one-third and caudal two-thirds of the PSN. Labeled neurons were large (30–50 μm), round-to-pear shaped multipolar cells with dendrites oriented primarily in the mediolateral direction. At the electron microscopic level, HRP reaction product was found throughout the cytoplasm of soma and processes of PSN projection neurons. The ultrastructural characteristics of these cells included a round, centrally placed nucleus and invaginated nuclear envelope, sparse Nissl bodies, numerous free ribosomes, mitochondria, lysosomes and Golgi complexes. Three to four main stem dendrites gradually tapered from the cell body and numerous synaptic terminals impinged upon soma and dendrites of labeled PSN neurons. Microiontophoretic injection of tritiated amino acids or HRP into the dorsal one-third of the PSN resulted in moderately dense terminal labeling in XII bilaterally, although mainly ipsilaterally. Terminal labeling was found diffusely throughout all regions of XII. Fibers descended the brainstem in the dorsolateral reticular formation and entered XII ventrolaterally. At the electron microscopic level, boutons containing HRP reaction product were found to synapse on dendritic processes in XII. Labeled boutons were characterized by clear, spherical vesicles and an asymmetrical postsynaptic density. The significance of these results are discussed in relation to oro-lingual motor behavior.
Similar content being viewed by others
Abbreviations
- Am:
-
Nucleus ambiguus
- Ax:
-
Axon
- Den:
-
Dendrite
- dlRF:
-
dorsolateral reticular formation
- G:
-
Golgi apparatus
- IO:
-
Inferior olive
- ITR:
-
Intertrigeminal region
- IV:
-
Fourth ventricle
- Lf:
-
Lipofuscin
- LRN:
-
Lateral reticular nucleus
- mRF:
-
Medial reticular formation
- mPB:
-
Medial parabrachial nucleus
- MV:
-
Motor trigeminal nucleus
- MVN:
-
Medial vestibular nucleus
- Nu:
-
Nucleus
- PSN:
-
Principal sensory trigeminal nucleus
- Py:
-
Pyramid
- R:
-
Ribosomes
- RF:
-
Reticular formation
- SC:
-
Subnucleus caudalis
- SI:
-
Subnucleus interpolaris
- SO:
-
Subnucleus oralis
- SOL:
-
Solitary tract nucleus
- STR:
-
Supratrigeminal region
- T:
-
Terminal
- TB:
-
Trapezoid body
- VII:
-
Facial nucleus
- VIIn:
-
Facial nerve
- X:
-
Dorsal vagal nucleus
- XII:
-
Hypoglossal nucleus
References
Aldes LD (1980) Afferent projections to the hypoglossal nucleus in the rat and cat. Anat Rec 196: 7A
Aldes LD (1982) Afferent and efferent connections of the somatic motor cortex related to tongue control in the rat. Neuroscience Abst 57: 3
Aldes LD, Boone TB (1984) A combined flat-embedding, HRP histochemical method for correlative light and electron microscopic study of single neurons. J Neurosci Res 11: 27–34
Astrom KE (1953) On the central course of afferent fibers in the trigeminal, facial, glossopharyngeal, and vagal nerves and their nuclei in the mouse. Acta Physiol Scand (Suppl) 106: 207–320
Barnard JW (1940) The hypoglossal complex of vertebrates. J Comp Neurol 72: 489–524
Belford GR, Killackey HP (1979) Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol 183: 305–322
Bodian D (1960) Electron microscopy: Two major synaptic types on spinal motoneurons. Science 151: 1093–1094
Boone TB, Aldes LD (1984) The ultrastructure of two distinct neuron populations in the hypoglossal nucleus of the rat. Exp Brain Res 54: 321–326
Boone TB, Aldes LD (1984) Synaptology of the hypoglossal nucleus of the rat. Exp Brain Res 57: 22–32
Borke RC, Nau ME, Ringler RL Jr (1983) Brain stem afferents of hypoglossal neurons in the rat. Brain Res 269: 47–55
Brodal A, Szabo T, Torvik A (1956) Corticofugal fibers to sensory trigeminal nuclei and nucleus of solitary tract. An experimental study in the cat. J Comp Neurol 106: 527–556
Cajal y Ramón S (1909) Histologie du système nerveux de l'homme et des vertébrés. Maloine, Paris
Clark WB, Bowsher D (1962) Terminal distribution of primary afferent trigeminal fibers in the rat. Exp Neurol 6: 372–383
Cooper MM (1981) The hypoglossal nucleus of the primate: a Golgi study. Neurosci Lett 21: 249–254
Darian-Smith I (1966) Neural mechanisms of facial sensation. Int Rev Neurobiol 9: 301–395
Darian-Smith I (1973) The trigeminal system. In: Iggo A (ed) Handbook of sensory physiology, Vol 2, Somatosensory system. Springer, Berlin, pp 271–314
Darian-Smith I, Mayday G (1960) Somatotopic organization within the brain-stem trigeminal complex of the cat. Exp Neurol 2: 290–309
Darian-Smith I, Proctor R, Ryan RD (1963) A single-neuron investigation of somatotopic organization within the cat's trigeminal brain-stem nuclei. J Physiol (Lond) 168: 147–157
Darian-Smith I, Yokota T (1966) Corticofugal effects on different neuron types within the cat's brain-stem activated by tactile stimulation of the face. J Neurophysiol 29: 185–206
Dubner R (1967) Interaction of peripheral and central input in the main sensory trigeminal nucleus of the cat. Exp Neurol 17: 186–201
Dubner R, Sessle BJ, Storey AT (1978) Jaw, facial and tongue reflexes. In: Dubner R, Sessle BJ, Storey AT (eds) The neural basic of oral and facial function. Plenum Press, New York, pp 290–310
Eccles JC (1964) The physiology of synapses. Academic Press, New York
Eisenman J, Landgren S, Novin D (1963) Functional organization in the main sensory trigeminal nucleus and in the rostral subdivision of the nucleus of the spinal trigeminal tract in the cat. Acta Physiol Scand Suppl 214 55: 1–44
Fukishima T, Kerr FWL (1979) Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol 183: 169–184
Gobel S (1971) Structural organization in the main sensory trigeminal nucleus. In: Dubner R, Kawamura (eds) Oral-facial sensory and motor mechanisms. Appleton-Century-Crofts, New York, pp 183–204
Gobel S, Dubner R (1969) Fine structural studies of the main sensory trigeminal nucleus in the cat and rat. J Comp Neurol 137: 459–494
Green JD, Negishi K (1963) Membrane potentials in hypoglossal motoneurons. J Neurophysiol 26: 835–856
Hayashimoto I (1960) Comparative anatomical studies of the hypoglossal nucleus of the carnivore. Bull Kobe Med Coll 20: 394–408
Holstege G, Kuypers HGJM (1977) Propriobulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. I. An anterograde degeneration study in the cat. Brain 100: 239–264
Holstege G, Kuypers HGJM, Decker JJ (1977) The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiographic tracing study in the cat. Brain 100: 265–286
Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt — glucose oxidase method. Brain Res 175: 341–346
Jacquin MF, Sembra K, Egger MD, Rhoades RW (1983) Organization of HRP-labeled trigeminal mandibular primary afferent neurons in the rat. J Comp Neurol 215: 397–420
Jones EG (1975) Possible determinants of the degree of retrograde neuronal labelling with horseradish peroxidase. Brain Res 85: 249–253
Kerr FWL, Kruger L, Schwassmann JO, Stern R (1968) Somatotopic organization of mechanoreceptor unit in the trigeminal nuclear complex of the macaque. J Comp Neurol 134: 127–144
Kievit J, Kuypers HGJM (1977) Organization of thalamocortical connexions of the frontal lobe in the rhesus monkey. Exp Brain Res 29: 299–332
Kruger L (1971) A critical review of theories concerning the organization of the sensory trigeminal nuclear complex of the brainstem. In: Dubner R, Kawamura Y (eds) Oral-facial sensory and motor mechanisms. Appleton-Century-Crofts, New York, pp 135–158
Kruger L, Michal F (1962a) A morphological and somatotopic analysis of single unit activity in the trigeminal sensory complex of the cat. Exp Neurol 5: 139–156
Kruger L, Michal F (1962b) A single neuron buccal cavity representation in the sensory trigeminal complex of the cat. Arch Biol 7: 491–503
Kuypers HGJM (1958) An anatomical analysis of corticobulbar connections to the pons and lower brain stem in the cat. J Anat 92: 198–218
Kuypers HGJM (1960) Central cortical projections to motor and somatosensory cell groups. Brain 83: 161–184
Lowe AA (1978) Excitatory and inhibitory inputs to hypoglossal motoneurons and adjacent reticular neurons in cats. Exp Neurol 62: 30–47
Lund RD, Webster KE (1967) Thalamic afferents from the spinal cord and trigeminal nuclei. An experimental anatomical study in the rat. J Comp Neurol 130: 313–318
Malmgren L, Olsson Y (1978) A sensitive method for histochemical demonstration of horseradish peroxidase in neurons following retrograde axonal transport. Brain Res 148: 279–294
Mesulam MM (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neuronal afferents and efferents. J Histochem Cytochem 26: 106–117
Mizuno N, Sauerland EK (1970) Trigeminal proprioceptive projections to the hypoglossal nucleus and the cervical central gray column. J Comp Neurol 139: 215–226
Morimoto T, Katao I, Kawamura Y (1966) Studies on functional organization of the hypoglossal nucleus. J Osaka Dent Univ 6: 75–87
Nord SG (1967) Somatotopic organization of the spinal trigeminal nucleus, the dorsal column nuclei and related structures in the cat. J Comp Neurol 130: 343–356
Nord SG (1968) Receptor field characteristic of single cells in the rat spinal trigeminal complex. Exp Neurol 21: 236–243
Nord SG, Ross GS (1973) Responses of trigeminal units in the monkey bulbar lateral reticular formation to noxious and non-noxious stimulation of the face: experimental and theoretical considerations. Brain Res 58: 385–399
Odutola AB (1976) Cell grouping and Golgi architecture of the hypoglossal nucleus of the rat. Exp Neurol 52: 356–371
Sakumoto T, Nagai T, Kimura H, Maeda T (1980) Electron microscopic visualization of tetramethylbenzidine reaction product on horseradish peroxidase histochemistry. Cell Molec Biol 26: 211–216
Smith RL (1973) The ascending fiber projections from the principal sensory trigeminal nucleus in the rat. J Comp Neurol 148: 423–446
Smith TG, Wuerker RB, Frank K (1967) Membrane impedance changes during synaptic transmission in cat spinal moto-neurons. J Neurophysiol 30: 1072–1096
Stewart WA, King RB (1963) Fiber projections from the nucleus caudalis of the spinal trigeminal nucleus. J Comp Neurol 121: 271–286
Streit P, Ruby JC (1977) A new and sensitive staining method for axonally transported horseradish peroxidase (HRP) in the pigeon visual system. Brain Res 126: 530–537
Szentágothai (1948) Anatomical considerations of monosynaptic reflex arcs. J Neurophysiol 11: 445–454
Tokado M, Yamamoto T, Hirose G (1982) On the supranuclear controls of the hypoglossal nucleus: a morphological study. Brain Nerve 34: 129
Torvik A (1956) Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. J Comp Neurol 106: 51–142
Torvik A (1957) The ascending fibers from the main trigeminal sensory nucleus. An experimental study in the cat. Am J Anat 100: 1–4
Travers JB, Norgren R (1983) Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220: 280–298
Uchizuno K (1965) Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207: 642–643
Valverde F (1961) Reticular formation of the pons and medulla: a Golgi study. J Comp Neurol 116: 71–99
Wall PD, Taub A (1962) Four aspects of the trigeminal nucleus and a paradox. J Neurophysiol 25: 110–126
Wan XST, Trojanowski JQ, Gonatas JO, Liu CN (1982) Cytoarchitecture of the extranuclear and commissural dendrites of hypoglossal nucleus neurons as revealed by conjugation of horseradish peroxidase with cholera toxin. Exp Neurol 78: 167–175
Woodburne RT (1936) A phylogenetic consideration of the primary and secondary centers and connections of the trigeminal complex in a series of vertebrates. J Comp Neurol 65: 403–501
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aldes, L.D., Boone, T.B. Organization of projections from the principal sensory trigeminal nucleus to the hypoglossal nucleus in the rat: an experimental light and electron microscopic study with axonal tracer techniques. Exp Brain Res 59, 16–29 (1985). https://doi.org/10.1007/BF00237661
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237661