Summary
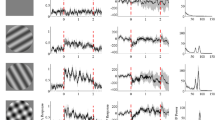
In the lateral geniculate nucleus (LGN) of paralyzed cats under urethane anaesthesia, an extensive disinhibitory region (DIR) outside the inhibitory surround of the receptive field (RF) was found in both sustained and transient cells. Its extent is comparable to that of McIlwain's periphery effect (1964). The responses to a light spot, flashed into different parts of the RF center, were used to assess the effect of different background patterns, located over the DIR, on responsiveness and receptive field organization. A straight line background cutting across the RF center led to a marked shrinkage in RF size and to a suppression of the center response. In sustained cells, these influences were not due to the light flux of the background, but were mainly due to the spatial property of the line itself. This was demonstrated by comparing the effect of a straight line background with that of a zigzag line or of distributed dots. The light flux for the different patterns and their spatial weighting was the same, so that they differed from each other solely in their form. A straight line background elicited much stronger suppression of the center response and more marked shrinkage of the RF than if the component dots are dispersed over a wider area, but keeping the radial distances of the individual dots from the RF-center constant. The data suggest that the dispersion of the component dots in different backgrounds plays an important role as response amplitude and RF diameter increase proportional to the dispersive area of the background patterns. For transient cells, all backgrounds used showed similar effects on center responses and RF diameter, indicating that for them it was the light flux of the backgrounds rather than their spatial property that caused the effects.
Similar content being viewed by others
References
Baumgartner G, Brown JL, Schulz A (1965) Responses of single units of the cat visual system to rectangular stimulus pattern. J Neurophysiol 28: 1–18
Bishop PO, Kozak W, Levick WR, Vakkur GJ (1962) The determination of the projection of the visual field on the lateral geniculate nucleus in the cat. J Physiol (Lond) 163: 503–539
Cleland BG, Lee BB, Vidyasagar TR (1983) Response of neurons in the cat's lateral geniculate nucleus to moving bars of different length. J Neurosci 3: 108–116
Cleland BG, Levick WR, Sanderson KJ (1973) Properties of sustained and transient ganglion cells in cat retina. J Physiol (Lond) 228: 649–680
Creutzfeldt OD, Nothdurft HC (1978) Representation of complex visual stimuli in the brain. Naturwissenschaften 65: 307–318
Daniels JD, Normal JL, Pettigrew JD (1977) Biases of oriented moving bars in lateral geniculate nucleus neurons of normal and stripe-reared cats. Exp Brain Res 29: 155–172
Daw NW, Pearlman AL (1969) Cat colour vision: one cone process or several? J Physiol (Lond) 201: 745–764
De Valois RL (1971) Contribution of different lateral geniculate cell types to visual behaviour. Vision Res Suppl No. 3, 383-396
Dreher B, Sanderson KJ (1973) Receptive field analysis: response to moving contours by single lateral geniculate neurons in the cat. J Physiol (Lond) 234: 95–118
Fernald R, Chase E (1971) An improved method for plotting retinal landmarks and focusing the eyes. Vision Res 11: 95–96
Fischer B, Krüger J (1974) The shift effect in the cat's lateral geniculate neurons. Exp Brain Res 21: 225–227
Hammond P, James CR (1971) The Purkinje shift in cat: extent of mesopic range. J Physiol (Lond) 216: 99–109
Hubel DH (1963) Integrative processes in central visual pathways of the cat. J Opt Soc Am 53: 58–66
Hubel DH, Wiesel T (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 160: 106–154
Kozak W, Rodieck RW, Bishol PO (1965) Responses of single units in lateral geniculate nucleus of cat to moving visual patterns. J Neurophysiol 28: 19–47
Lee BB, Creutzfeldt OD, Elepfandt A (1979) The responses of magno-and parvocellular cells of the monkey's lateral geniculate body to moving stimuli. Exp Brain Res 35: 547–557
Lee BB, Virsu V, Creutzfeldt OD (1977) Responses of cells in the cat lateral geniculate nucleus to moving stimuli at various levels of light and dark adaptation. Exp Brain Res 27: 51–59
Li CY, Chang YJ, Hsu HC (1983) Nonconcentric receptive field organization of single units in the cat's lateral geniculate nucleus. Biochem Biophys (Beijing) 2: 46–48
Maffei L, Fiorentini A (1972) Retinogeniculate convergence and analysis of contrast. J Neurophysiol 35: 65–72
McIlwain JT (1964) Receptive fields of optic tract axons and lateral geniculate cells: peripheral extent and barbiturate sensitivity. J Neurophysiol 27: 1154–1173
McIlwain J, Creutzfeldt OD (1967) Microelectrode study of synaptic excitation and inhibition in the lateral geniculate nucleus of the cat. J Neurophysiol 30: 1–21
Singer W, Creutzfeldt OD (1970) Reciprocal lateral inhibition of on-and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res 10: 311–330
Valberg A, Lee BB, Tigwell DA, Creutzfeldt OD (1985) A simultaneous contrast effect of steady remote surrounds on responses of cells in macaque lateral geniculate nucleus. Exp Brain Res 58: 604–608
Vidyasagar TR (1984) Contribution of inhibitory mechanisms to the orientation sensitivity of cat dLGN neurons. Exp Brain Res 55: 192–195
Vidyasagar TR, Urbas JV (1982) Orientation sensitivity of cat LGN neurons with and without inputs from visual cortical areas 17 and 18. Exp Brain Res 46: 157–169
Virsu V, Lee BB, Creutzfeldt OD (1977) Dark adaptation and receptive field organization of cells in the cat lateral geniculate nucleus. Exp Brain Res 27: 35–50
Wiesel TN, Hubel DH (1966) Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol 29: 1115–1156
Author information
Authors and Affiliations
Additional information
Supported by the Science Fund of the Chinese Academy of Sciences (No. 850161) to C.Y.L.
Rights and permissions
About this article
Cite this article
Li, C.Y., He, Z.J. Effects of patterned backgrounds on responses of lateral geniculate neurons in cat. Exp Brain Res 67, 16–26 (1987). https://doi.org/10.1007/BF00269448
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00269448