Summary
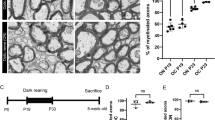
The quantitative effects of dark-rearing and light exposure on the ultrastructural characteristics of synapses and synaptic boutons in layer 4 of the rat visual cortex (area 17) have been investigated using stereological techniques. Two experimental groups (each containing 5 animals) were investigated i) animals dark-reared upto weaning at 21 days post natum (21DPN) and then light exposed until 52DPN (Group 21/31), and ii) littermate animals totally dark-reared until 52DPN (Group 52dD). The results indicate a significantly higher mean density of synapses in the neuropil of layer 4 in group 21/31 (3.58×108 · mm-3) compared with group 52dD (2.68×108 · mm-3). Although the density per unit volume of synapses with identified asymmetrical synaptic membrane specialisations was not significantly different in group 21/31 than in group 52dD (but was significantly lower than animals reared normally), the density of synapses with identified symmetrical synaptic membrane specialisations was about 200% higher in group 21/31 versus group 52dD. However, significant differences were detected in the number of asymmetrical synapses established by single synaptic boutons in group 21/31 (1.21 ± 0.11) compared with group 52dD (1.10 ± 0.09). On the basis of the numbers of post-synaptic targets contacted by an individual synaptic bouton, a significantly higher density of synaptic boutons was found in group 21/31 (2.32×108 · mm-3) compared with group 52dD (1.82×108 · mm-3). Furthermore, planar quantitative data indicated significant inter-group differences in the ultrastructure of asymmetrical and symmetrical synaptic boutons. The results of this study provide evidence indicating marked structural alterations in the synaptic connectivity of layer 4 of the rat visual cortex following the light exposure of rats dark-reared upto weaning. Indeed visual deprivation severely affected the ‘inhibitory’ circuitry in the major thalamorecipient territory of the visual cortex.
Similar content being viewed by others
References
Abercrombie M (1953) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247
Beaulieu C, Colonnier M (1983) The number of neurons in the different laminae of the binocular and monocular regions of area 17 in the cat. J Comp Neurol 217: 337–344
Beaulieu C, Colonnier M (1986) The effects of impoverished and enriched environments on the number and size of boutons containing flat vesicles in the visual cortex of cat. Soc Neurosci Abstr I. 36.7 p 128
Bear MF, Schmechel DE, Ebner FF (1985) Glutamic acid decarboxylase in the striate cortex of normal and monocularlydeprived kittens. J Neurosci 5: 1262–1275
Blue ME, Parnavelas JG (1983) The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol 12: 697–712
Borges S, Berry M (1976) Preferential orientation of stellate cell dendrites in the visual cortex of dark-reared rats. Brain Res 112: 141–147
Borges S, Berry M (1978) The effects of dark-rearing on the development of the visual cortex of the rat. J Comp Neurol 180: 277–300
Chronwall B, Wolff JR (1980) Prenatal and postnatal development of GABA-accumulating cells in the occipital cortex of rat. J Comp Neurol 190: 187–208
Colonnier M, Beaulieu C (1985) The differential effect of impoverished and enriched environments on the number of ‘round asymmetrical’ and ‘flat symmetrical’ synapses in the visual cortex of cat. Soc Neurosci Abstr II. 68.5 p226
Couteaux R, Akert K, Heuser JE, Reese TS (1977) Ultrastructural evidence for vesicle exocytosis. Neurosci Res Prog Bull 15: 603–607
Cragg BG (1967) Changes in visual cortex on first exposure to light. Nature (Lond) 215: 251–253
Cruz-Orive L-M (1978) Particle size-shape distributions. The general spheroid problem. II. Stochastic model and practical guide. J Microscopy 112: 153 et seq.
deGroot D, Bierman A (1983) The complex-shaped ‘perforated’ synapse, a problem in quantitative stereology of the brain. J Microsc 131: 355–360
deGroot DMG, Bierman EPB (1986) A critical evaluation of methods for estimating the numerical density of synapses. J Neurosci Methods 18: 79–101
Fairen A, Peters A, Saldanha J (1977) A new approach for examining Golgi-impregnated neurons by lightand electronmicroscopy. J Neurocytol 6: 311–337
Feldman ML, Peters A (1978) The forms of non-pyramidal neurons in the visual cortex of the rat. J Comp Neurol 179: 761–794
Fifkova E (1970) The effect of monocular deprivation on the synaptic contacts of the visual cortex. J Neurobiol 1: 285–294
Fifkova E (1970) Changes of axosomatic synapses in the visual cortex of monocularly deprived rats. J Neurobiol 1, 3: 61–71
Freund TF, Martin KAC, Somogyi P, Whitteridge D (1985) Innervation of cat visual areas 17 and 18 by physiologically identified X-and Y-type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J Comp Neurol 242: 275–291
Gabbott PL, Stewart MG, Rose SPR (1981) Quantitative synaptic architecture in the visual system of dark-reared rats: an approach using inter-active image analysis. J Anat 133: C46
Gabbott PL, Stewart MG, Rose SPR (1986) The quantitative effects of dark-rearing and light exposure on the laminar distribution of neurons and glia in the visual cortex (area 17) of the rat. Exp Brain Res 64: 225–232
Gabbott PLA, Stewart MG (1987) Distribution of neurons and glia in the visual cortex (area 17) of the adult albino rat: a quantitative description. Neuroscience (in press)
Gray EG (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex. An electron microscope study. J Anat 93: 420–433
Gundersen HJG (1977) Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc 111: 219–223
Hendry SHC, Jones EG, Kennedy MB (1985) Modulation of GABA, substance P, and protein kinase immunoreactivities in monkey striate cortex following eye removal. Soc Neurosci Abstr 11: 16
Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L (1977) Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol 81: 275–300
Jack JJB, Noble D, Tsien RW (1975) Electric current flow in excitable cells. Clarendon Press, Oxford
Mayhew TM (1979) Stereological approach to the study of synapse morphometry with particular regard to estimating number in a volume and on a surface. J Neurocytol 8: 121–138
Miller M (1981) Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. J Neurocytol 10: 859–878
Müller L, Pattiselanno A, Vrensen G (1981) The postnatal development of the presynaptic grid in the visual cortex of rabbits and the effects of dark-rearing. Brain Res 205: 39–48
Orban GA (1984) Neuronal operations in the visual cortex. Studies in brain function. 11. Springer, Berlin Heidelberg New York Tokyo
Parnevelas JG, Luder R, Pollard SG, Sullivan K, Lieberman AR (1983) A qualitative and quantitative ultrastructural study of glial cells in the developing visual cortex of the rat. Philos Trans R Soc (Lond) B 301: 55–84
Peters A, Kaiserman-Abramof IR (1969) The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch 100: 467–506
Peters A, Palay SL, Webster HdeF (1976) The fine structure of the nervous system. Saunders, Philadelphia
Peters A, Proskauer CC, Feldman M, Kimerer L (1979) The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. V. Degenerating axon terminals synapsing with Golgi-impregnated neurons. J Neurocytol 8: 331–357
Peters A (1985) The visual cortex of the rat. In: Peters A, Jones EG (eds). Visual cortex, Vol 3. Cerebral cortex, Vol 3. Plenum Press, New York, pp 19–80
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17: 208–212
Ribak CE (1978) Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol 7: 461–478
Ribak CE, Robertson RT (1986) Effects of neonatal monocular enucleation on the number of GAD-positive puncta in rat visual cortex. Exp Brain Res 62: 203–206
Riccio RV, Matthews MA (1985) The postnatal development of the rat primary visual cortex during optic nerve impulse blockade by intraocular tetrodotoxin: a quantitative electron microscopic analysis. Dev Brain Res 20: 55–68
Ryugo R, Ryugo DK, Killackey H (1975) Differential effects of enucleation on two populations of layer V pyramidal neurons. Brain Res 88: 554–559
Sherman SM, Spear PD (1982) Organisation of visual pathways in normal and visually deprived cats. Physiol Rev 62, 2: 738–855
Sillito AM (1984) Functional considerations of the operation of GABAergic inhibitory processes in the visual cortex. In: Jones EG, Peters A (eds) Cerebral cortex, Vol 2. Functional properties of cortical cells. Plenum Press, New York, pp 91–117
Somogyi P, Hodgson AJ (1985) Antiserum to γ-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem 33: 249–257
Underwood EE (1977) Quantitative stereology. Addison Wesley, Reading MA
Valverde F (1967) Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res 3: 337–352
Valverde F (1967) Aspects of cortical organisation related to the shape of neurons with intra-cortical axon arbors. J Neurocytol 5: 509–529
Valverde F, Ruiz-Marcos A (1967) Dendritic spines in the visual cortex of the mouse. Introduction to a mathematical model. Exp Brain Res 8: 269–283
Vrensen G, DeGroot D (1975) The effects of monocular deprivation on synaptic terminals in the visual cortex of rabbits. A quantitative electron microscope study. Brain Res 93: 15–24
Vrensen G, Nunes Cardozo J, Müller L, Van Der Want J (1980) The presynaptic grid: a new approach. Brain Res 184: 23–40
Vrensen G (1980) Visual experience and modification of the presynaptic grid in the visual cortex of rabbits. ‘Cellular analogues of conditioning and neural plasticity’. Physiol Sci 36: 75–85
Vrensen G, Nunes-Cardozo J (1981) Changes in the size and shape of synaptic connections after visual training: an ultrastructural approach to synaptic plasticity. Brain Res 218: 79–97
Weibel ER (1979) Stereological methods, Vol 1. Practical methods for biological morphometry. Academic Press, London
Winfield DA (1983) The postnatal development of synapses in the different laminae of the visual cortex in the normal kitten and in kittens with eyelid suture. Dev Brain Res 9: 155–169
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gabbott, P.L.A., Stewart, M.G. Quantitative morphological effects of dark-rearing and light exposure on the synaptic connectivity of layer 4 in the rat visual cortex (area 17). Exp Brain Res 68, 103–114 (1987). https://doi.org/10.1007/BF00255237
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00255237