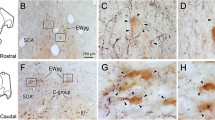
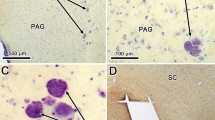
Summary
The colocalization of neurotensin- and cholecystokinin-like immunoreactivities was demonstrated in neurons of the ventral mesencephalon of the rat by using a double-labeling indirect immunofluorescence procedure for the simultaneous detection of two antigens in the same tissue section. Greater than 90% of the neurotensin-positive perikarya distributed throughout the ventral mid-brain (primarily located in the ventral tegmental area, medial substantia nigra, and rostral and caudal linear raphe nuclei) were found to also contain cholecystokinin immunoreactivity. Neurons single-labeled for either peptide were also present, with those immunoreactive for cholecystokinin alone far outnumbering those containing only neurotensin. By combining the double-labeling colocalization technique with fluorescence retrograde tracing, some of the forebrain projections of these neurons were determined. Ventral mesencephalic neurons containing both neurotensin and cholecystokinin were found to project to the nucleus accumbens, prefrontal cortex, or amygdala. The present results, combined with those of previous studies, suggest that there are complex heterogeneous subpopulations of presumed dopaminergic ventral mesencephalic neurons which give rise to the ascending mesotelencephalic systems and which may contain both neurotensin and cholecystokinin, either peptide alone, or neither of these two peptides.
Similar content being viewed by others
References
Anden N-E, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67: 313–326
Andrade R, Aghajanian GK (1981) Neurotensin selectively activates dopaminergic neurons of the substantia nigra. Soc Neurosci Abstr 7: 573
Bissette G, Nemeroff CB, Loosen PT, Prange AJ Jr, Lipton MA (1976) Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature 262: 607–609
Clineschmidt BV, McGuffin JC, Bunting PB (1979) Neurotensin: antinocisponsive action in rodents. Eur J Pharmacol 54: 129–139
Coons AH (1958) Fluorescent antibody methods. In: Danielli JF (ed) General cytochemical methods. Academic Press, New York, pp 399–422
Crawley JN, Stivers JA, Blumstein LK, Paul SM (1985) Cholecystokinin potentiates dopamine-mediated behaviors: evidence for modulation specific to a site of coexistence. J Neurosci 5: 1972–1983
De Witte P, Swanet E, Gewess M, Goldman S, Roques B, Vanderhaeghen J-J (1985) Psychopharmacological profile of cholecystokinin using the self-stimulation and the drug discrimination paradigm. Ann NY Acad Sci 448: 470–487
Dunn AJ, Snijders R, Hurd RW, Kramarcy NR (1982) Induction of catelepsy by central nervous system administration of neurotensin. Ann NY Acad Sci 400: 345–353
Ervin GN, Birkemo LS, Nemeroff CB, Prange AJ Jr (1981) Neurotensin blocks certain amphetamine-induced behaviors. Nature 291: 73–76
Fallon JH, Koziell DA, Moore RY (1978) Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol 180: 509–532
Fallon JH, Moore RY (1978a) Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and periform cortex. J Comp Neurol 180: 533–544
Fallon JH, Moore RY (1978b) Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180: 545–580
Fallon JH, Hicks R, Loughlin SE (1983) The origin of cholecystokinin terminals in the basal forebrain of the rat: evidence from immunofluorescence and retrograde tracing. Neurosci Lett 37: 29–35
Fallon JH, Seroogy KB (1985) Forebrain projections from mid-brain cholecystokinin-containing neurons in the rat. Ann NY Acad Sci 448: 596–597
Fallon JH, Loughlin SE (1985) Substantia nigra. In: Paxinos G (ed) The rat nervous system, Vol 1. Forebrain and midbrain. Academic Press, Sydney, pp 353–374
Frey P (1985) Changes in cholecystokinin content in rat brain after subchronic treatment with neuroleptics. Ann NY Acad Sci 448: 601–603
Fuxe K, Andersson K, Locatelli V, Agnati LF, Hökfelt T, Skirboll L, Mutt V (1980) Cholecystokinin peptides produce marked reduction of dopamine turnover in discrete areas in the rat brain following intraventricular injection. Eur J Pharmacol 67: 329–331
Goedert M, Iversen SD, Emson PC (1985) The effects of chronic neuroleptic treatment on neurotensin-like immunoreactivity in the rat central nervous system. Brain Res 335: 334–336
Govoni S, Hong JS, Yang H-YT, Costa E (1980) Increase of neurotensin content elicited by neuroleptics in nucleus accumbens. J Pharmacol Exp Ther 215: 413–417
Hamilton M, Sheehan MJ, De Belleroche J, Herberg LJ (1984) The cholecystokinin analogue, caerulein, does not modulate dopamine release or dopamine-induced locomotor activity in the nucleus accumbens of the rat. Neurosci Lett 44: 77–82
Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M (1984) Occurrence of neurotensinlike immunoractivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol 222: 543–559
Hökfelt T, Skirboll L, Rehfeld JF, Goldstein M, Markey K, Dann O (1980) A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience 5: 2093–2124
Jennes L, Stumpf WE, Kalivas PW (1982) Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol 210: 211–224
Jurna I, Zetler G (1981) Antinociceptive effect of centrally administered caerulein and cholecystokinin octapeptide (CCK-8). Eur J Pharmacol 73: 323–331
Kalivas PW (1984) Neurotensin in the ventromedial mesencephalon of the rat: anatomical and functional considerations. J Comp Neurol 226: 495–507
Kalivas PW, Miller JS (1984) Neurotensin neurons in the ventral tegmental area project to the medial nucleus accumbens. Brain Res 300: 157–160
Katsuura G, Itoh S (1982) Sedative action of cholecystokinin octapeptide on behavioral excitation by thyrotropin releasing hormone and methamphetamine in the rat. Jpn J Physiol 32: 83–91
Lindvall O, Björklund A (1983) Dopamine- and norepinephrine-containing neuron systems: their anatomy in the rat brain. In: Emson PC (ed) Chemical neuroanatomy. Raven Press, New York, pp 229–255
Luttinger D, Frye GD, Bissette G (1982) Effects of neurotensin on the actions of barbiturates and ethanol. Ann NY Acad Sci 400: 259–267
McCarthy PS, Walker RJ, Yajima H, Kitagawa K, Woodruff GN (1979) The action of neurotensin on neurones in the nucleus accumbens and cerebellum of the rat. Gen Pharmacol 10; 331–333
Moore RY, Bloom FE (1978) Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Ann Rev Neurosci 1: 129–169
Morley JE, Levine AS, Lindblad S (1981) Intraventricular cholecystokinin-octapeptide produces hypothermia in rats. Eur J Pharmacol 74: 249–251
Nair NPV, Lal S, Bloom DM (1986) Cholecystokinin and schizophrenia. Prog Brain Res 65: 237–258
Nemeroff CB, Bissette G, Prange AJ Jr, Loosen FT, Lipton MA (1977) Neurotensin: central nervous system effects of a hypothalamic peptide. Brain Res 128: 485–496
Nemeroff CB, Bissette G, Manberg PJ, Osbahr III AJ, Breese GR, Prange AJ Jr (1980) Neurotensin-induced hypothermia: evidence for an interaction with dopaminergic systems and the hypothalamic-pituitary thyroid axis. Brain Res 195: 69–84
Nemeroff CB, Hernandez DE, Luttinger D, Kalivas PW, Prange AJ Jr (1982) Interactions of neurotensin with brain dopamine systems. Ann NY Acad Sci 400: 330–344
Nemeroff CB, Luttinger D, Hernandez DE, Mailman RB, Mason GA, Davis SD, Widerlov E, Frye GD, Kilts CA, Beaumont K, Breese GR, Prange AJ Jr (1983) Interactions of neurotensin with brain dopamine systems: biochemical and behavioral studies. J Pharmacol Exp Ther 225: 337–345
Nemeroff CB (1986) The interaction of neurotensin with dopaminergic pathways in the central nervous system: basic neurobiology and implications for the pathogenesis and treatment of schizophrenia. Psychoneuroendocrinology 11: 15–37
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, Sydney New York London
Pinnock RD (1985) Neurotensin depolarizes substantia nigra dopamine neurones. Brain Res 338: 151–154
Reches A, Burke RE, Jiang D, Wagner HR, Fahn S (1982) The effect of neurotensin on dopaminergic neurons in rat brain. Ann NY Acad Sci 400: 420–421
Schmued LC, Fallen JH (1986) Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377: 147–164
Schneider LH, Alpert JE, Iversen SD (1983) CCK-8 modulation of mesolimbic dopamine: antagonism of amphetamine-stimulated behaviors. Peptides 4: 749–753
Seroogy KB, Dangaran K, Lim S, Fallon JH (1985a) Innervation of forebrain structures by ventral mesencephalic neurons containing both cholecystokinin- and tyrosine hydroxylase-like immunoreactivities: a fluorescent triple-labeling study. Soc Neurosci Abstr 11: 145
Seroogy KB, Fallon JH, Loughlin SE, Leslie FM (1985b) Few cortical cholecystokinin immunoreactive neurons have long projections. Exp Brain Res 59: 533–542
Skirboll LR, Grace AA, Hommer DW, Rehfeld J, Goldstein M, Hökfelt T, Bunney BS (1981) Peptide-monoamine coexistence: studies of the actions of cholecystokinin-like peptide on the electrical activity of midbrain dopamine neurons. Neuroscience 6: 2111–2124
Snijders R, Kramarcy NR, Hurd RW, Nemeroff CB, Dunn AJ (1982) Neurotensin induces catalepsy in mice. Neuropharmacology 21: 465–468
Studler JM, Simon H, Cesselin F, Legrand JC, Glowinski J, Tassin JP (1981) Biochemical investigation on the localization of the cholecystokinin octapeptide in dopaminergic neurons originating from the Ventral tegmental area of the rat. Neuropeptides 2: 131–139
Studler JM, Reibaud M, Tramu G, Blanc G, Glowinski J, Tassin JP (1984) Pharmacological study on the mixed CCK8/DA meso-nucleus accumbens pathway: evidence for the existence of storage sites containing the two transmitters. Brain Res 298: 91–97
Sugimoto T, Itoh K, Yasui Y, Kaneko T, Mizuno N (1985) Coexistence of neuropeptides in projection neurons of the thalamus in the cat. Brain Res 347: 381–384
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde and immunofluorescence study in the rat. Brain Res Bull 9: 321–353
Uhl GR, Goodman RR, Snyder SH (1979) Neurotensin-containing cell bodies, fibers and nerve terminals in the brain stem of the rat: immunohistochemical mapping. Brain Res 167: 77–91
Uhl GR, Kuhar MJ, Snyder SH (1977) Neurotensin: immunohistochemical localization in rat central nervous system. Proc Natl Acad Sci USA 74: 4059–4063
Ungerstedt U (1971) Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand (Suppl) 367: 1–48
Van Ree JM, Gaffori O, De Wied D (1983) In rats, the behavioral profile of CCK-8-related peptides resembles that of antipsychotic agents. Eur J Pharmacol 93: 63–78
Van Ree JM, De Wied D (1985) Neuroleptic-like activity and antipsychotic action of cholecystokinin-related peptides. Ann NY Acad Sci 448: 671–673
Voigt MM, Wang RY, Westfall TC (1985) The effects of cholecystokinin on the in vivo release of newly synthesized [3H] dopamine from the nucleus accumbens of the rat. J Neurosci 5: 2744–2749
White FJ, Wang RY (1984) Interactions of cholecystokinin octapeptide and dopamine on nucleus accumbens neurons. Brain Res 300: 161–166
Widerlov E, Breese GR (1982) Actions of neurotensin on dopaminergic and serotonergic pathways in rat brain. Ann NY Acad Sci 400: 428–430
Widerlov E, Kalivas PW, Lewis MH, Prange AJ Jr, Breese GR (1983) Influence of cholecystokinin on central monoaminergic pathways. Reg Peptides 6: 99–109
Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M (1985) Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience 14: 427–453
Zetler G (1981) Central depressant effects of caerulein and cholecystokinin octapeptide (CCK-8) differ from those of diazepam and haloperidol. Neuropharmacology 20: 277–283
Zetler G (1982) Cholecystokinin octapeptide, caerulein and caerulein analogues: effects on thermoregulation in the mouse. Neuropharmacology 21: 795–801
Zetler G (1983) Neuroleptic-like effects of ceruletide and cholecystokinin octapeptide: interactions with apomorphine, methylphenidate and picrotoxin. Eur J Pharmacol 94: 261–270
Zetler G (1985) Neuropharmacological profile of cholecystokinin-like peptides. Ann NY Acad Sei 448: 448–469
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seroogy, K.B., Mehta, A. & Fallon, J.H. Neurotensin and cholecystokinin coexistence within neurons of the ventral mesencephalon: projections to forebrain. Exp Brain Res 68, 277–289 (1987). https://doi.org/10.1007/BF00248793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248793