Summary
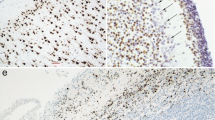
In freshly fixed biopsies of human cerebral cortex obtained at surgery, immunocytochemical staining with antibodies against tyrosine hydroxylase (the rate limiting biosynthetic enzyme for catecholamines) revealed, in addition to a dense axonal plexus, a population of immunoreactive cell bodies. The neuronal nature of these cells was ascertained by: i) the presence of a rich rough endoplasmic reticulum in the cell body and of synapses on the cell body and dendrites, and ii) the demonstration of the lack of reactivity with the astroglial marker, glial fibrillary acidic protein, in the tyrosine hydroxylase-immunoreactive cells. The tyrosine hydroxylase-immunoreactive neurons were found in all areas of cortex sampled, and were located almost exclusively in the infragranular layers. Most tyrosine hydroxylase-immunoreactive cells were bipolar and were vertically oriented, but a few had a multipolar or horizontal dendritic arbor. The dendrites of these cells were varicose and aspiny, and the axons were very thin. Tyrosine hydroxylase-immunoreactive neurons were reported to be present transiently in the developing mammalian cerebral cortex and only recently in cerebral cortex of mature mammalian brains. Internuncial neurons in the human cerebral cortex containing a catecholamine synthesizing enzyme would be significant, in particular considering that catecholamines are likely to be involved in some major mental disorders.
Similar content being viewed by others
References
Berger B, Verney C, Alvarez C, Vigny A, Helle KB (1985a) New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analyses. Neuroscience 15: 983–998
Berger B, Verney C, Gaspar P, Febvret A (1985b) Transient expression of tyrosine hydroxylase immunoreactivity in some neurons of the rat neocortex during postnatal development. Dev Brain Res 23: 141–144
De Lima AD, Singer W (1986) Cholinergic innervation of the cat striate cortex: a choline acetyltransferase immunocytochemical analysis. J Comp Neurol 250: 324–338
Dubach M, Schmidt R, Kunkel D, Bowden DM, Martin R, German DC (1987) Primate neostriatal neurons containing tyrosine hydroxylase: immunohistochemical evidence. Neurosci Lett 75: 205–210
Eckenstein F, Thoenen H (1983) Cholinergic neurons in the rat cerebral cortex demonstrated by immunohistochemical localization of choline acetyltransferase. Neurosci Lett 36: 211–215
Fallon JH, Loughlin SE (1987) Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. In: Jones EG, Peters A (eds) Cerebral cortex, Vol 6. Plenum, New York, pp 41–127
Foster GA, Schlutzberg M, Dahl D, Goldstein M, Verhofstad AAJ (1985) Ephemeral existence of a single catecholamine synthetic enzyme in the olfactory placode and the spinal cord of the embryonic rat. Int J Dev Neurosci 3: 597–608
Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C (1987) Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett 80: 257–262
Glowinski J, Tassin JP, Thierry AM (1984) The mesocorticoprefrontal dopaminergic neurons. Trends Neurosci 7: 415–418
Hedreen JC, Bacon SJ, Cork LC, Kitt CA, Crawford GD, Salvaterra PM, Price DL (1983) Immunocytochemical identification of cholinergic neurons in the monkey central nervous system using monoclonal antibodies against choline acetyltransferase. Neurosci Lett 43: 173–177
Hendry SHC, Jones EG, Killackey HP, Chalupa LM (1987) Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Dev Brain Res 37: 313–317
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577–580
Iacovitti L, Lee J, Joh TH, Reis DJ (1987) Expression of tyrosine hydroxylase in neurons of cultured cerebral cortex: evidence for phenotypic plasticity in neurons of the CNS. J Neurosci 7: 1264–1270
Jaeger CB, Joh TH (1983) Transient expression of tyrosine hydroxylase in some neurons of the developing inferior colliculus of the rat. Brain Res 11: 128–132
Jaeger CB, Ruggiero DA, Albert VR, Park DH, Joh TH, Reis DJ (1984) Aromatic L-amino acid decarboxylase in the rat brain: immunocytochemical localization in neurons of the brain stem. Neuroscience 11: 691–713
Joh TH, Ross ME (1983) Preparation of catecholamine synthesizing enzymes as immunogens for immunocytochemistry. In: Cuellon AC (ed) IBRO Handbook series, methods in neurosciences, Vol 3. John Wiley and Sons, Oxford, pp 121–138
Kosaka T, Hama K, Nagatsu I (1987a) Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp Brain Res 68: 393–405
Kosaka T, Kosaka K, Hatagushi I, Wu J-Y, Ottersen OP, Storm-Mathiesen J, Homa K (1987b) Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 66: 191–210
Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH (1987) The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 7: 279–290
Meister B, Hökfelt T, Steinbusch HWM, Skagerberg G, Lindvall O, Geffard M, Joh TH, Cuello AC, Goldstein M (1988) Do tyrosine hydroxylase-immunoreactive neurons in the ventrolateral arcuate nucleus produce dopamine or only L-Dopa? J Chem Neuroanatomy 1: 59–64
Morrison JH, Foote SL, O'Connor D, Bloom FE (1982) A laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-β-hydroxylase immunocytochemistry. Brain Res Bull 9: 309–319
Park JK, Joh TH, Ebner FF (1986) Tyrosine hydroxylase is expressed by neocortical neurons after transplantation. Proc Natl Acad Sci USA 83: 7495–7498
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17: 208–211
Snyder SM (1982) Neurotransmitters and CNS disease: schizophrenia. Lancet 2: 970–974
Specht LA, Pickel VM, Joh TH, Reis DJ (1981) Light-microscopic immunocytochemical localization of tyrosine-hydroxylase in prenatal rat brain. II. Late ontogeny. J Comp Neurol 199: 255–276
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hornung, J.P., Törk, I. & De Tribolet, N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp Brain Res 76, 12–20 (1989). https://doi.org/10.1007/BF00253618
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00253618