Summary
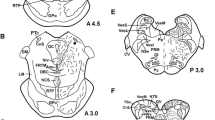
The paraventricular nucleus (PVN) of male albino rats was analyzed for the presence of glucocorticoid receptor-like immunoreactivity (GR-LI) in neuropeptide containing neurons. Using immunohistochemistry, coronal sections trough the entire PVN were double-stained with a mouse monoclonal antibody against GR and one of the following antisera: rabbit antiserum to corticotropin releasing factor (CRF), neurotensin (NT), enkephalin (ENK), cholecystokinin (CCK), thyrotropin releasing hormone (TRH), galanin (GAL), peptide histidine isoleucine (PHI), vasoactive intestinal polypeptide (VIP), somatostatin (SOM) or tyrosine hydroxylase (TH). For comparison the occurrence of GR-LI in NT-, SOM-, NPY- or TH-positive neurons of the arcuate nucleus was also studied. Our results indicate that GR-LI is present in the parvocellular part of the PVN but not in its magnocellular portion. Virtually every parvocellular neuron in the PVN containing one of the above mentioned peptides was also positive for GR, with the exception of SOM neurons, of which only about two thirds showed detectable levels of GR-LI. All TH-positive, presumably dopamine neurons in the PVN were GR-positive. In the arcuate nucleus all TH- and NPY-positive neurons as well as a large proportion of the SOM- and NT-immunoreactive neurons contained GR-LI. The results indicate that in the PVN, in addition to the CRF neurons, certain peptidergic neurons in the parvocellular part of the PVN, without any established role in the control of ACTH synthesis and release, may also be under glucocorticoid control. This seems to be the case also for most arcuate neurons.
Similar content being viewed by others
References
Agnati LF, Fuxe K, Yu Z-Y, Härfstrand A, Okret S, Wikström A-C, Goldstein M, Zoli M, Vale W, Gustafsson J-Å (1985) Morphometrical analysis of the distribution of corticotrophin releasing factor, glucocorticoid receptor and phenylethanolamine-N-methyltransferase-immunoreactive structures in the paraventricular hypothalamic nucleus of the rat. Neurosci Lett 54:147–152
Antoni FA, Palkovits M, Makara GB, Linton EA, Lowry PJ, Kiss JZ (1983) Immunoreactive corticotropin-releasing hormone in the hypothalamo-infundibular tract. Neuroendocrinology 36:415–423
Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson J-Å (1988) Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci USA 85:9331–9335
Blake CA (1974) Stimulation of pituitary prolactin and TSH release in lactating and proestrus rats. Endocrinology 94:503–508
Boyd AE III, Reichlin S (1978) Neural control of prolactin secretion in man. Psychoneuroendocrinology 3:113–130
Brown MR, Rivier C, Gray TS (1988) Thyrotropin-releasing factor (TRF) effects on pituitary ACTH release and autonomic function. Soc Neurosci Abstr 14:1287
Buckingham JC (1982) Secretion of corticotrophin and its hypothalamic releasing factor in response to morphine and opioid peptides. Neuroendocrinology 35:111–116
Buckingham JC (1986) Stimulation and inhibition of corticotrophin releasing factor secretion by beta endorphin. Neuroendocrinology 42:148–152
Burlet A, Tonon MC, Dreyfuss F, Tankosic P (1984) La colocalization des peptides neurohypophysaires et de la corticolibérine dans le cerveau de rat. Annis Endocr 45:189–199
Ceccatelli S, Eriksson M, Hökfelt T (1989) Distribution and coexistence of corticotropin-releasing factor-, neurotensin-, enkephalin-, cholecystokinin-, galanin- and vasoactive intestinal polypeptide/peptide histidine isoleucine-like peptides in the parvocellular part of the paraventricular nucleus. Neuroendocrinology 49:309–323
Chen HT, Meites J (1975) Effects of biogenic amines and TRH on the release of prolactin and TSH in the rat. Endocrinology 96:10
Cintra A, Fuxe K, Härfstrand A, Agnati LF, Wikström A-C, Okret S, Vale W, Gustafsson J-Å (1987) Presence of glucocorticoid receptor immunoreactivity in corticotrophin releasing factor and in growth hormone releasing factor immunoreactive neurons of the rat di- and telencephalon. Neurosci Lett 77:25–30
Cintra A, Fuxe K, Wikström A-C, Gustafsson J-Å (1989) Evidence for glucocorticoid receptor and thyrotropin-releasing hormone costoring neurons in various preoptic and hypothalamic nuclei of the male rat. Brain Res (in press)
Coons AH (1958) Fluorescent antibody methods. In: Danielli JF (ed) General cytochemical methods. Academic Press, New York, pp 399–422
Ducommun P, Sakiz E, Guillemin R (1966a) Liability of plasma TSH levels in the rat in response to non specific exteroceptive stimuli. Proc Soc Exp Biol Med 121:921–923
Ducommun P, Sakiz E, Guillemin R (1966b) Dissociation of the acute secretions of thyrotropin and adrenocorticotropin. Am J Physiol 210:1257–1259
Ducommun P, Vale W, Sakiz E, Guillemin R (1967) Reversal of the inhibition of TSH secretion due to acute stress. Endocrinology 80:953–956
Du Ruisseau P, Taché Y, Selye H, Ducharme JR, Collu R (1977) Effect of chronic stress on pituitary hormone release induced by combined hemi-extirpation of the thyroid, adrenal and ovary in rats. Neuroendocrinology 24:169–182
Elde R, Hökfelt T, Johansson O, Schultzberg M, Efendić S, Luft R (1978) Cellular localization of somatostatin. Metabolism 27:1151–1159
Everitt BJ, Meister B, Hökfelt T, Melander T, Terenius L, Rökaeus Å, Theodorsson-Norheim E, Dockray G, Edwardson J, Cuello C, Elde R, Goldstein M, Hemmings H, Ouimet C, Walaas I, Greengard P, Vale W, Weber E, Wu J-Y (1986) The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res Rev 11:97–155
Fahrenkrug J, Pedersen JH (1984) Development and validation of a specific radioimmunoassay for PHI in plasma. Clin Chim Acta 143:183–192
Fahrenkrug J, Schaffalitzky de Muckadell OB (1977) Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med 89:1379–1388
Fahrenkrug J, Schaffalitzky de Muckadell OB (1978) Distribution of vasoactive intestinal polypeptide (VIP) in the porcine central nervous system. J Neurochem 31:1445–1451
Frey P (1985) Changes in cholecystokinin content in rat brain after subchronic treatment with neuroleptics. In: Vanderhaeghen JJ, Crawley J (eds) Neuronal cholecystokinin. Ann NY Acad Sci, New York, pp 601–633
Fuxe K, Hökfelt T, Löfström A, Johansson O, Agnati L, Everitt B, Goldstein M, Jeffcoate S, White N, Eneroth P, Gustafsson J-Å (1976) On the role of neurotransmitters and hypothalamic hormones and their interactions in hypothalamic and extrahypothalamic control of pituitary function and sexual behavior. In: Naftolin F, Ryan KJ, Davies J (eds) Subcellular mechanisms in reproductive neuroendocrinology. Elsevier, Amsterdam, pp 193–246
Fuxe K, Wikström A-C, Okret S, Agnati LF, Härfstrand A, Yu Z-Y, Granholm L, Zoli M, Vale W, Gustafsson J-Å (1985) Mapping of glucocorticoid receptor imnrunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology 117:1803–1812
Fuxe K, Cintra A, Härfstrand A, Agnati LF, Kalia M, Zoli M, Wikström A-C, Okret S, Aronsson, Gustafsson, J-Å (1987) Central glucocorticoid receptor immunoreactive neurons: new insight into the endocrine regulation of the brain. In: Ganong WF, Dallman MF, Roberts JL (eds) The hypothalamic-pituitary-adrenal axis revisited. Ann NY Acad Sci (New York) 512:362–393
Fuxe K, Cintra A, Aronsson M, Agnati LF, Kitayama I, Wikström A-C, Okret S, Gustafsson J-Å (1988) Localization and distribution of glucocorticoid receptor immunoreactivity and of glucocorticoid receptor mRNA in the rat brain using immunocytochemistry and in situ hybridization. In: Imura et al. (eds) Progress in endocrinology. Elsevier, Amsterdam, pp 875–883
Gudelsky GA, Berry S, Meltzer HY (1988) Neurotensin activates tuberoinfundibular dopamine neurons and increases serum corticosterone concentrations. Soc Neurosci Abstr 14:1287
Gustafsson J-Å, Carlstedt-Duke J, Poellinger L, Okret S, Wikström A-C, Brönnegård M, Gillner M, Dong Y, Fuxe K, Cintra A, Härfstrand A, Agnati L (1987) Biochemistry, molecular biology, and physiology of the glucocorticoid receptor. Endocr Rev 8:185–234
Hashimoto K, Patt J, Brodish A (1977) Hypothalamic influence on stress-induced plasma levels of pituitary hormones. Fed Proc Fed Am Soc Exp Biol 36:322 (Abstr)
Hökfelt T, Fahrenkrug J, Tatemoto K, Mutt V, Werner S, Hulling A-L, Terenius L, Chang KJ (1983) The PHI(PHI-27)/corticotropin-releasing factor/enkephalin-immunoreactive hypothalamic neurons: possible morphological basis of integrated control of prolactin, corticotropin, and growth hormone secretion. Proc Natl Acad Sci USA 80:895–898
Hökfelt T, Fahrenkrug J, Ju G, Ceccatelli S, Tsuruo Y, Meister B, Mutt V, Rundgren M, Brodin E, Terenius L, Hulling A-L, Werner S, Björklund H, Vale W (1987) Analysis of peptide histidine-isoleucine- and vasoactive intestinal polypeptide-immunoreactive neurons in the central nervous system with special reference to their relation to CRF- and enkephalin-like immunoreactivities in the paraventricular hypothalamic nucleus. Neuroscience 23:827–857
Jingami H, Matsukura S, Numa S, Imura H (1985) Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticolropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/β-lipotropin precursor mRNA in the pituitary in rals. Endocrinology 117:1314–1320
Jobin M, Ferland L, Labrie F (1976) Effect of pharmacological blockade of ACTH and TSH secretion on the acute stimulation of prolactin release by exposure to cold and ether stress. Endocrinology 99:146–151
Johnson DG, de C Nogueira Araujo GM (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Meth 43:349
Kato Y, Iwasaki I, Iwasaki J, Abe H, Yanahara N (1978) Prolactin release by vasoactive intestinal polypeptide in rats. Endocrinology 103:554–558
Kiss JZ, Mezey E, Skirboll L (1984) Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci USA 81:1854–1858
Kovacs K, Kiss JZ, Makara JB (1986) Glucocorticoid implants around the hypothalamic paraventricular nucleus prevent the increase of corticotropin-releasing factor and arginine vasopressin immunostaining induced by adrenalectomy. Neuroendocrinology 44:229–234
Krulich L, Illner P (1973) Effect of stress on plasma levels of LH, FSH, prolactin, TSH and GH in normal male rats. Fed Proc Fed Am Soc Exp Biol 32:281 (Abstr)
Lightman SL, Young III WS (1987) Changes in hypothalamic preproENK A mRNA following stress and opiate withdrawal. Nature 328:643–645
Lind RW, Swanson LW, Chin DA, Bruhn TO, Ganten D (1984) Angiotensin II: an immunohistochemical study of its distribution in the paraventriculo-hypophysial system and its co-localization with vasopressin and CRF in parvocellular neurons. Neurosci Abstr 10:88
Liposits Z, Uht RM, Harrison RW, Gibbs FP, Paull WK, Bohn MC (1987) Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic neurons synthesizing corticotropin-releasing factor (CRF). Histochemistry 87:407–412
Lundberg JM, Terenius L, Hökfelt T, Tatemoto K (1984) Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci 4:2376–2386
MacLeod RM, Lehmeyer JE (1974) Studies on the mechanism of dopamine-mediated inhibition of prolactin secretion. Endocrinology 94:1077–1085
Markey KA, Kondo S, Shenkman L, Goldstein M (1980) Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17:79–85
Matsumura M, Yamanoi A, Yamamoto S, Saito S (1983) In vivo and in vitro effects of cholecystokinin octapeptide on the release of β-endorphin-like immunoreactivity. Neuroendocrinology 36:443–448
McEwen BS, Weiss JM, Schwartz LS (1969) Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res 16:227–241
Meister B, Hökfelt T (1988) Peptide- and transmitter-containing neurons in the mediobasal hypothalamus and their relation to GABAergic systems: possible roles in control of prolactin and growth hormone secretion. Synapse 2:585–605
Meister B, Ceccatelli S, Hökfelt T, Andén N-E, Andén M, Theodorsson E (1989) Neurotransmitters, neuropeptides and binding sites in the rat mediobasal hypothalamus: effects of monosodium glutamate (MSG) lesions. Exp Brain Res 76:343–368
Merchenthaler I, Vigh S, Petrusz P, Schally AV (1982) Immunocytochemical localization of corticotropin-releasing factor (CRF) in the brain. Am J Anat 165:385–396
Mezey E, Reisine TD, Skirboll L, Beinfeld M, Kiss JZ (1986) Role of cholecystokinin in corticotropin release: coexistence with vasopressin and corticotropin-releasing factor in cells of the rat hypothalamic paraventricular nucleus. Proc Natl Acad Sci USA 83:3510–3512
Mueller GP, Twohy CP, Chen HT, Avdis JP, Meites J (1976) Effects of L-tryptophan and restraint stress on hypothalamic and brain serotonin turnover, and pituitary TSH and prolactin release in rats. Life Sci 18:715–724
Mühlen A, Von Zur, Lammers M, Kobberling J, Hesch RD (1974) TSH, T3 and corticosterone in rats under various environmental conditions and after L-T3 and D-T3 administration. Endocr Exp (Bratislava) 8:237
Nairn RC (Ed.) (1969) Fluorescent protein tracing, 3rd edn. E & S Livingstone Ltd, Edinburgh London
Okret S, Wikström A-C, Wrange Ö, Andersson B, Gustafsson J-Å (1984) Monoclonal antibodies against the rat liver glucocorticoid receptor. Proc Natl Acad Sci USA 81:1609–1613
Paull WK, Gibbs FP (1983) The corticotropin releasing factor (CRF) neurosecretory system in intact, adrenalectomized, and adrenalectomized-dexamethasone treated rats. Histochemistry 78:303–316
Piekut DT, Joseph SA (1985) Relationship of CRF-immunostained cells and magnocellular neurons in the paraventricular nucleus of rat hypothalamus. Peptides 6:873–882
Plotsky PM, Sawchenko PE (1987) Hypophysial-portal plasma levels, median eminence content, and immunohistochemical staining of corticotropin-releasing factor, arginine vasopressin, and oxytocin after pharmacological adrenalectomy. Endocrinology 120:1361–1369
Rivier C, Brown M, Vale W (1977) Effect of neurotensin, substance P and morphine sulfate on the secretion of prolactin and growth hormone in the rat. Endocrinology 100:751
Sawchenko PE, Swanson LW, Vale WW (1984a) Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci 4:1118–1129
Sawchenko PE, Swanson LW, Vale WW (1984b) Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci USA 81:1883–1887
Sawchenko PE (1987) Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide and steroid specificity. J Neurosci 7:1093–1106
Schultzberg M, Lundberg JM, Hökfelt T, Terenius L, Brandt J, Elde RP, Goldstein M (1978) Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience 3:1669–1186
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Swanson LW, Sawchenko PE, Lind RW (1986) Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: implications for the stress response. Progr in Brain Res 68:169–190
Swanson LW (1987) The hypothalamus. In: Björklund A, Hökfelt T, Swanson LW (eds) Handbook of chemical neuroanatomy, Vol 5. Integrated systems of the CNS, Part I. Elsevier, Amsterdam, pp 1–124
Tilders F, Tatemoto K, Berkenbosch F (1984) The intestinal peptide PHI-27 potentiates the action of corticotropin-releasing factor on ACTH release from rat pituitary fragments in vitro. Endocrinology 115:1633–1635
Tramu G, Pillez A, Leonardelli J (1978) An efficient method of antibody elution for the successive or simultaneous location of two antigens by immunocytochemistry. J Histochem Cytochem 26:322–324
Tramu G, Beauvillain JC, Croix D, Pillez A, Garaud JC (1984) Coexistence of hypothalamic factors with other neuropeptides: demonstration in the median eminence of rats and guinea-pigs. Neurochem Int 6:721–730
Uht RM, McKelvy JF, Harrison RW, Bohn MC (1988) Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res 19:405–411
Vale W, Blackwell R, Grant G, Guillemin R (1973) TRF and thyroid hormones in prolactin secretion by rat anterior pituitary cells in vitro. Endocrinology 93:26
Vijayan E, McCann SM (1979) In vivo and in vitro effects of substance P and neurotensin on gonadotropin and prolactin release. Endocrinology 105:64–68
Visser TJ, Klootwijik W, Doctor R, Henneman G (1977) A different approach to the radioimmunoassay and thyrotropin-releasing hormone. In: Radioimmunoassay and related procedures in medicine. National Atomic Energy Agency, Vienna, pp 469–477
Wessendorf MW, Elde RP (1985) Characterization of an immunofluorescence technique for the demonstration of coexisting neurotransmitters within nerve fibers and terminals. J Histochem Cytochem 331:984–994
Westendorf JM, Philips MA, Schonbrunn A (1983) Vasoactive intestinal peptide stimulates hormone release from corticotrophic cells in culture. Endocrinology 112:550
Young III WS, Mezey E, Siegel RE (1986) Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett 70:198–203
Zamboni I, De Martine C (1967) Buffered picric acid formaldehyde: a new rapid fixative for electron microscopy. J Cell Biol 35:148A
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ceccatelli, S., Cintra, A., Hökfelt, T. et al. Coexistence of glucocorticoid receptor-like immunoreactivity with neuropeptides in the hypothalamic paraventricular nucleus. Exp Brain Res 78, 33–42 (1989). https://doi.org/10.1007/BF00230684
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230684