Summary
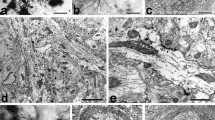
By means of tracing with the lectin Phaseolusvulgaris leucoagglutinin (PHA-L), we examined in the thalamus of the mouse, the axon terminals of fibers originating in the barrel cortex. Vibratome sections of the brain were subjected to PHA-L immunocytochemistry and processed for light and electron microscopy. We observed small (0.5–0.8 μm in diameter) varicosities of labeled fibers in the nucleus ventrobasalis (VB) and the nucleus posterior (PO) as well as labeled giant terminals (3–5 μm in diameter) in PO. The analysis involved examination of serial sections and computer-aided reconstruction of several terminals. The small varicosities in VB appear to be small axon terminals forming distinct asymmetric synapses with small dendritic profiles. Some labeled terminals are apposed to, but not synaptically related with, the cell bodies of neurons in VB that are retrogradely labeled with PHA-L. The small varicosities seen with the light microscope in PO are terminals forming asymmetric synapses with dendritic shafts. The giant terminals in PO appear as large, vesicle-filled profiles forming part of synaptic glomeruli, i.e. complexes of one corticothalamic terminal engulfing several excrescences of a single dendrite. A giant terminal forms several asymmetric synapses (about 8) with these excrescences, as well as numerous (up to 15) puncta adhaerentia. The glomeruli are enveloped in glial lamellae, and they are often found at the bifurcations of primary dendritic segments. We suggest that the small terminals in VB are in the service of feedback signalling from the barrel cortex to its principal thalamic relay nucleus; the functional importance of this projection may reside in increased spatio-temporal discrimination. We interpret the giant terminals in PO as elements serving feed-forward processing, allowing the barrel cortex to influence, via PO, parts of the motor pathway modulating the animal's ongoing behavior.
Similar content being viewed by others
References
Aldes LD (1988) Thalamic connectivity of rat somatic, motor cortex. Brain Res Bull 20:333–348
Blackstad TW, Kjaerheim A (1961) Special axo-dendritic synapses in the hippocampal cortex: electron and light microscopic studies on the layer of mossy fibers. J Comp Neurol 117:133–159
Campbell G, Frost DO (1988) Synaptic organization of anomalous retinal projections to the somatosensory and auditory thalamus: target-controlled morphogenesis of axon terminals and synaptic glomeruli. J Comp Neurol 272:383–408
Campos-Ortega J, Glees P, Neuhoff V (1968) Ultrastructural analysis of individual layers in the lateral geniculate nucleus of the monkey. Z Zellforsch 87:82–100
Carvell GE, Simons DJ (1987) Thalamic and corticocortical connections of the second somatic sensory area of the mouse. J Comp Neurol 265:409–427
Cadusseau J (1987) Proposition d'une nouvelle définition du noyau postérieur du thalamus chez le rat, d'aprés une étude hodologique. Thése de doctorat d'état, Université de Poitiers, Poitiers, France
Colonnier M, Guillery RW (1964) Synaptic organization in the lateral geniculate nucleus of the monkey. Z Zellforsch Mikr Anat 62:333–355
Cullen MJ, Kaiserman-Abramof IR (1976) Cytological organization of the dorsal lateral geniculate nuclei in mutant anophthalmic and postnatally enucleated mice. J Neurocytol 5:1375–1393
Famiglietti EV, Peters A (1972) The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 144:285–334
Friedrich VL Jr, Mugnaini E (1981) Electron microscopy: Preparation of neural tissues for electron microscopy. In: Heimer L, RoBards MJ (eds) Neuroanatomical tract-tracing methods. Plenum Press, New York, pp 345–375
Groenewegen HJ, Wouterlood FG (1990) Light and electron microscopic tracing of neuronal connections with Phaseolus vul garis-leucoagglutinin (PHA-L), and combinations with other neuroanatomical techniques. In: Björklund A, Hökfelt T, Wouterlood FG, Van den Pol AN (eds) Analysis of neuronal microcircuits and synaptic interactions. Handbook of chemical neuroanatomy, Vol 8. Elsevier Biomedical Press, Amsterdam, pp 47–124
Guillery RW (1969) The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch 96:1–38
Hajdu F, Hassler R, Somogyi G (1982) Neuronal and synaptic organization of the lateral geniculate nucleus of the tree shrew, Tupaia glis. Cell Tissue Res 224:207–223
Hoogland PV, Welker E, Van der Loos H (1987) Organization of the projections from barrel cortex to thalamus in mice studied with Phaseolus vulgaris-leucoagglutinin and HRP. Exp Brain Res 68:73–87
Hoogland PV, Welker E, Van der Loos H, Wouterlood FG (1988) The organization and structure of the thalamic afferents from the barrel cortex in the mouse: a PHA-L study. In: Bentivoglio M, Spreafico R (eds) Cellular thalamic mechanisms. Elsevier Science Publishers, Amsterdam, pp 151–162
Jones EG, Powell TPS (1969) Electron microscopy of synaptic glomeruli in the thalamic relay nuclei of the cat. Proc R Soc B 172:153–171
Jones EG, Rockel AJ (1971) The synaptic organization in the medial geniculate body of afferent fibers ascending from the inferior colliculus. Z Zellforsch Mikrosk Anat 113:44–66
Karlsson U (1967) Three-dimensional studies of neurons in the lateral geniculate nucleus of the rat. III. Specialized neuronal contacts in the neuropil. J Ultrastruct Res 17:137–157
Lieberman AR (1974) Comments on the fine structural organization of the dorsal lateral geniculate nucleus of the mouse. Z Anat Entwickl-Gesch 145:261–267
Ma W, Peschanski M, Ralston III HJ (1987) The differential synaptic organization of the spinal and lemniscal projections to the ventrobasal complex of the rat thalamus. Evidence for convergence of the two systems upon single thalamic neurons. Neuroscience 22:925–934
Mason A, Robson JA (1979) Morphology of retino-geniculate axons in the cat. Neuroscience 4:79–97
McMahan UJ (1967) Fine structure of synapses in the dorsal nucleus of the lateral geniculate body of normal and blinded rats. Z Zellforsch Mikr Anat 76:116–146
McAllister JP, Wells J (1981) The structural organization of the ventral posterolateral nucleus in the rat. J Comp Neurol 197:271–301
Peschanski M, Roudier F, Ralston III HJ, Besson J-M (1985) Ultrastructural analysis of the terminals of various somatosensory pathways in the ventrobasal complex of the rat thalamus: an electron-microscopic study using wheatgerm agglutinin conjugated to horseradish peroxidase as an axonal tracer. Somatosens Res 3:75–87
Peters A, Palay SL, Webster HdeF (1991) The fine structure of the nervous system. Saunders, Philadelphia, 3rd edn
Prada C (1987) Effect of light deprivation upon the morphology of axon terminals in the dorsal geniculate nucleus of the mouse: an electron microscopic study using serial sections. Neurosci Res 4:255–267
Rafols JA, Valverde F (1973) The structure of the dorsal lateral geniculate nucleus in the mouse: a Golgi and electron microscopic study. J Comp Neurol 150:303–332
Rapisardi SC, Miles TP (1984) Synaptology of retinal terminals in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 223:515–534
Robson JA, Hall WC (1977a) The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). I. Cytoarchitecture and connections. J Comp Neurol 173:355–388
Robson JA, Hall WC (1977b) The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). II. Synaptic organization and comparisons with the dorsal lateral geniculate nucleus. J Comp Neurol 173:389–416
Roger M, Cadusseau J (1987) Anatomical evidence of a reciprocal connection between the posterior thalamic nucleus and the parvocellular division of the red nucleus in the rat: a combined retrograde and anterograde study. Neuroscience 21:573–583
Rouiller EM, Welker E (1989) Structure of thalamic afferents from the auditory cortex of the rat, a PHA-L study. Neurosci Lett Suppl 2:261
Špaček J, Lieberman AR (1974) Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat (Lond) 117:487–516
Sternberger LA (1979) Immunocytochemistry. John Wiley & Sons, New York
Szentágothai J (1970) Glomerular synapse, complex synaptic arrangements, and their operational significance. In: Schmitt FO (ed) The neurosciences, second study program. The Rockefeller University Press, New York, pp 427–442
Szentágothai J, Hámori J (1969) Growth and differentiation of synaptic structures under circumstances of deprivation of function and of distant connections. Symp Intl Soc Cell Biol, Vol 8. Academic Press, New York, pp 301–320
Welker E, Hoogland PV, Van der Loos H (1988) Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res 73:411–435
Wise SP, Donoghue JP (1986) Motor cortex of rodents. In: Jones EG, Peters A (eds) Cerebral cortex, Vol 5. Sensory-motor areas and aspects of cortical connectivity. Plenum Press, New York, pp 243–270
Woolsey TA, Van der Loos H (1970) The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17:205–242
Wouterlood FG (1979) Light microscopic identification and photography of Golgi impregnated central nervous system neurons during sectioning for electron microscopy. Stain Technol 54:325–329
Wouterlood FG, Hoogland PV, Welker E, Van der Loos H (1990) Giant and small terminals in the thalamus labelled after injections of PHA-L in the barrel cortex of the mouse. An electron microscopic study. Eur J Neurosci Suppl 3:54
Wouterlood FG, Sauren YMHF, Pattiselanno A (1988) Compromises between penetration of antisera and preservation of ultrastructure in pre-embedding electron microscopic immunocytochemistry. J Chem Neuroanat 1:65–80
Yamakado M (1985) Postnatal development of barreloid neuropils in the ventrobasal complex of mouse thalamus: a histochemical study for cytochrome oxidase. Brain and Nerve 37:497–506
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoogland, P.V., Wouterlood, F.G., Welker, E. et al. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L). Exp Brain Res 87, 159–172 (1991). https://doi.org/10.1007/BF00228517
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228517