Abstract
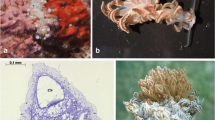
The multiple defensive strategies of four Mediterranean ascoglossan molluscan species, belonging to two different Polybranchioidea families, Cyerce cristallina (Trinchese, 1881) and Caliphylla mediterranea (Costa A., 1869) (Polybranchiidae), and Ercolania funerea (Costa A., 1867) and Placida dendritica (Alder and Hancock, 1843) (Stiligeridae), were studied. E. funerea, P. dendritica and C. mediterranea were collected from the lake Fusaro (Arco Felice, Naples) in 1992. C. cristallina was collected from Capo Miseno (Bay of Naples) in 1991. C. cristallina and E. funerea easily undergo autotomy of dorso-lateral appendages (cerata) followed by an extraordinarily quick (8 to 10 d) regeneration of the latter. Histological analyses showed the presence, at the basis of both normal and regenerated cerata of these species, of a muscular sphincter which facilitates the autotomic process. C. mediterranea and P. dendritica, which do not undergo autotomy, lack this anatomical feature but feed upon algae belonging to the Chaetomorpha and Bryopsis genera, and, as shown by electron microscopy studies, retain large quantities of chloroplasts which they use as camouflage amidst algae and to escape predation. E. funerea also exploits this behavioral defense mechanism. Histological investigations also revealed in the cerata of all four species several multi-cellular mucous glands responsible for the secretion of the slime typical for these molluscs. C. cristallina, E. funerea and P. dendritica secrete large amounts of slime, whose extracts displayed ichthyotoxic activity when assayed by the Gambusia affinis ichthyotoxicity assay, while extracts of C. mediterranea slime were not toxic. A chemical analysis of the slime, mantle and cerata led to the isolation of polypropionate α- and γ-pyrones from all species except C. mediterranea. These secondary metabolites possess structures that differ only by the degree of methylation and the geometry of double bonds of the side chain and are specifically distributed in cerata and slime of C. cristallina and E. funerea. The pyrones also display different activities in the Hydra vulgaris regeneration assay and in the G. affinis ichthyotoxicity test and, depending on their structural features and tissue distribution, are likely to play a role either as defense allomones or as supportive inducers of cerata regeneration. In conclusion, combined biological observations and histological and chemical techniques generated valuable information on the defensive behavior of these four ascoglossan species which exploit various combinations of the same behavioral and/or chemical defensive strategies and thus successfully avoid predation.
Similar content being viewed by others
References
Brown, J. L. (1975) The evolution of behaviour. W. W. Norton & Co. Inc., New York
Cimino, G., Crispino, A., Di Marzo, V., Gavagnin, M., Ros, J. D. (1990). Oxytoxins, bioactive molecules produced by the marine opisthobranch mollusc Oxynoe olivacea from a diet-derived precursor. Experientia 46: 767–770
Cimino, G., Sodano, G. (1989) The chemical ecology of Mediterranean opisthobranchs. Chem. Scr. 29: 389–394
Clark, K. B., Jensen, K. R., Stirts, H. (1990). Survey for functional kleptoplasty among West Atlantic Ascoglossa (Sacoglossa, Mollusca: Opisthobranchia). Veliger 33: 339–345
Coll, J. C., La Barre, S., Sammarco, P. W., Williams, F. T., Bakus, G. J. (1982). Chemical defences in soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef: a study of comparative toxicities. Mar. Ecol. Prog. Ser. 8: 271–278
Dawe, R. D., Wright, J. L. C. (1986). The major polypropionate metabolites from the sacoglossan mollusc Elysia chlorotica. Tetrahedron Lett. 27: 2559–2562
De Petrocellis, B., De Petrocellis, L., Maharajan, V., Quagliarotti, G., Minei, R. (1985). Stimulation of tentacle regeneration in Hydra by phorbol ester TPA. Acta Embryol. Morph. exp. 6: 51–59
De Petrocellis, L., Di Marzo, V., Arca', B., Gavagnin, G., Minei, R., Cimino, G. (1991). The effect of diterpenoidic diacylglycerols on tentacle regeneration in Hydra vulgaris. Comp. Biochem. Physiol. 100C: 603–607
De Petrocellis, L., Maharajan, V., De Petrocellis, B., Minei, R. (1986). Bud induction in decapitated Hydra attenuata by 5-azacytidine: a morphological study. J. Embryol. exp. Morph. 93: 105–119
Di Marzo, V., Vardaro, R. R., De Petrocellis, L., Villani, G., Minei, R., Cimino, G. (1991). Cyercenes, novel pyrones from the ascoglossan mollusc Cyerce cristallina. Tissue distribution, biosynthesis and possible involvement in defense and regenerative processes. Experientia 47: 1221–1227
Faulkner, D. J. (1992). Marine natural products. Nat. Product Rep. (Lond.), 9: 322–364
Faulkner, D. J., Ghiselin, M. T. (1983). Chemical defense and evolutionary ecology of Dorid nudibranchs and some other opisthobranch gastropods. Mar. Ecol. Prog. Ser. 13: 295–301
Graves, D. A., Gibson, M. A., Bleakney, J. S. (1979). The digestive diverticulae of Alderia modesta and Elysia chlorotica (Opisthobranchia: Sacoglossa). Veliger 21: 415–422
Greene, R. W., Muscatine, L. (1972). Symbiosis in sacoglossan opisthobranchs: photosynthetic products of animal-chloroplast associations. Mar. Biol. 14: 253–259
Gunthorpe, L., Camerom, A. (1987). Bioactive properties of extracts from Australian dorid nudibranchs. Mar. Biol. 94: 39–43
Hagadone, M. R., Burreson, B. J., Scheuer, P. J. (1979). Defense allomones of the nudibranch Phyllidia varicosa Lamarck 1801. Helv. chim. Acta 62: 2484–2494
Hay, M. E., Pawlik, J. R., Duffy, J. E., Fenical, W. (1989). Seaweedherbivore-predator interactions: host-plant specialization reduces predation on small herbivores. Oecologia 81: 418–427
Herdman, W. A., Clubb, J. A. (1890). Third report upon the nudibranchiata of the L.M.B.C. district. Proc. biol. Soc. Liverpool 3: 131–169
Hinde, R., Smith, D. C. (1974). Chloroplast symbiosis and the extent to which it occurs in Sacoglossa (Gastropoda: Mollusca). Biol. J. Linn. Soc. 6: 349–356
Ireland, C., Faulkner, J. (1981). The metabolites of the marine molluscs Tridachiella diomedea and Tridachia crispata. Tetrahedron 37: 233–240
Ireland, C., Scheuer, P. J. (1979). Photosynthetic marine mollusks: In vivo 14C incorporation into metabolites of the sacoglossan Placobranchus ocellatus. Science, N.Y. 205: 922–923
Karuso, P. (1987). Chemical ecology of the nudibranchs. In: Scheuer, P. J. (ed.) Bioorganic marine chemistry. I. Springer-Verlag, New York, p. 31–60
Ksebati, M. B., Schmitz, F. J. (1985). Tridachiapyrones: Polypropionate-derived metabolites from the sacoglossan mollusc Tridachia crispata. J. org. Chem. 50: 5637–5642
Lewin, R. A. (1970). Toxin secretion and tail autotomy by irritated Oxynoe panamensis. Pacif. Sci. 24: 356–358
Marin, A., Di Marzo, V., Cimino, G. (1991). A histological and chemical study of the cerata of the opisthobranch mollusc Tethys fimbria. Mar. Biol. 111: 353–358
Marin, A., Ros, J. D. (1988). Los sacoglosos (Mollusca: Opisthobranchia) del sudeste iberico. Catalogo de las especies y presencia de chloroplastos algales en las mismas. Iberus 8: 25–49
Marin, A., Ros, J. D. (1989). The chloroplast-animal association in four iberian sacoglossan opisthobranchs: Elysia timida, Elysia translucens, Thuridilla hopei and Boselia mimetica. Scientiea mar. 53: 429–440
Muscatine, L., Greene, R. W. (1973). Chloroplasts and algae as symbionts in molluscs. Int. Rev. Cytol. 36: 137–169
Ros, J. D. (1976). Sistemas de defensa en los opistobranquios. Oecologia aquat. (Barcelona), 2: 41–77
Roussis, V., Pawlik, J. R., Hay, M. E., Fenical W. (1990). Secondary metabolites of the chemically rich ascoglossan Cyerce nigricans. Experientia 46: 327–329
Sammarco, P. W., Coll, J. C. (1988). The chemical ecology of alcyonarian corals (Coelenterata: Octocorallia). In: Scheuer, P. J. (ed.) Bioorganic marine chemistry. 2. Springer Verlag, New York, p. 90–92
Schaller, H. C., Bodenmuller, H. (1981). Isolation and amino acid sequence of a morphogenetic peptide from hydra. Proc. natn. Acad. Sci. U.S.A. 78: 7000–7004
Schulte, G. R., Scheuer, P. J. (1982). Defense allomones of some marine mollusks. Tetrahedron 38: 1857–1863
Stasek, C. R. (1967). Autotomy in the Mollusca. Occ. Pap. Calif. Acad. Sci. 61: 1–44
Takabayashi, J., Takahashi, S. (1990). An allelochemical elicits arrestment in Aponteles Kariyei in feces of nonhost lareyi. J. Chem. Ecol. 16: 2009–2017
Taylor, D. L. (1968). Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis (Gastropoda: Opisthobranchia). J. mar. biol. Ass. U.K. 48: 1–15
Thompson, T. E. (1960). Defensive adaptations in opisthobranchs, J. mar. biol. Ass. U.K., 39: 123–134
Todd, C. (1981). The ecology of nudibranch molluscs. Oceanogr. mar. biol. A. Rev. 19: 141–234
Trench, R. K. (1975). Of “leaves that crawl”: functional chloroplasts in animal cells. Symp. Soc. exp. Biol. 29: 229–265
Vardaro, R. R., Di Marzo, V., Cimino, G. (1992a). Placidenes: cyercene-like polypropionate γ-pyrones from the Mediterranean ascoglossan mollusc Placida dendritica. Tetrahedron Lett. 33: 2875–2878.
Vardaro, R. R., Di Marzo, V., Crispino, A., Cimino, G. (1991). Cyercenes, novel polypropionate pyrones from the autotomizing Mediterranean mollusc Cyerce cristallina. Tetrahedron 47: 5569–5576
Vardaro, R. R., Di Marzo, V., Marin, A., Cimino, G. (1992b). α-and γ-pyrone-polypropionates from the Mediterranean ascoglossan mollusc Ercolania funerea. Tetrahedron 48: 9561–9566
Warmke, G. L., Almodovar, L. R. (1972). Observations on the life cycle and regeneration in Oxynoe antillarum, Morch, an ascoglossan opisthobranch from the Caribbean. Bull. mar. Sci. 22: 67–74
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Di Marzo, V., Marin, A., Vardaro, R.R. et al. Histological and biochemical bases of defense mechanisms in four species of Polybranchioidea ascoglossan molluscs. Marine Biology 117, 367–380 (1993). https://doi.org/10.1007/BF00349312
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349312