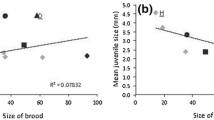
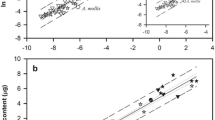
Abstract
Gammarus oceanicus Segerstråle, 1947 and Echinogammarus marinus (Leach, 1815) were sampled during the breeding season from Oslofjord in 1984, and their lipid composition examined in relation to reproductive condition. In G. oceanicus, female lipid content increased as the ovary matured. Both the amount of lipid stored and the rate of accumulation were greater in spring than in winter. Spring eggs contained 12.4 μg lipid, of which 63% was triacylglycerol and 27% phospholipid. Both fractions decreased steadily during embryonic development. Winter eggs contained 19.2μg lipid, of which 52% was triacylglycerol and 43% phospholipid. During the early stages of embryonic development the amount of phospholipid decreased sharply, whereas that of triacylglycerol increased, suggesting that some of the fatty acid released from phospholipid was sequestered temporarily as triacylglycerol. When newly spawned, both winter and spring eggs were richer in monoenoic fatty acids than adult amphipods and these acids were the major fuel consumed during development. ω6 fatty acids were utilised more slowly than ω3 acids, and egg carotenoid pigment content remained constant. Female E. marinus increased in lipid content as the ovary matured. Spring eggs contained 14.7 μg lipid when newly spawned and this increased to 16.6 μg during the early stages of development. This increase was entirely triacylglycerol, which declined in later stages; the source of the extra lipid was unclear. Eggs contained very little phospholipid or sterol, and both of these components remained at a steady low level during development. E. marinus eggs were not significantly rich in thonoenoic acids compared with adults, and saturated, monoenoic and polyenoic acids were utilised about equally during development. Both adults and eggs were rich in 20.4ω6, which was utilised at a slower rate than the ω3 polyunsaturated acids during embryonic development; again, egg carotenoid pigment content remained constant. In both species there was a decrease in the size of the egg (and as a result, of the newly hatched juvenile), but an increase in total reproductive output (i.e., the total weight of the egg clutch) per female as the breeding season proceeded. The reproductive output of an individual female is probably related to food availability during the period of ovarian maturation, whereas the size of an individual egg is dictated largely by feeding conditions for the juveniles once they are independent of the female. The different patterns of lipid utilisation during development found in this study emphasize the flexibility of response in the reproductive biology of gammarid amphipods. It is not yet possible, however, to relate the differing patterns in a simple way either to egg size or total female reproductive output. Two outstanding problems are the source of extra triacylglycerol during the early stages of development of E. marinus and the metabolic cost of brooding eggs.
Similar content being viewed by others
Literature cited
Ackman, R. G. and R. D. Burgher: Cod liver oil fatty acids as secondary reference standards in the GLC of polyunsaturated fatty acids of animal origin: analysis of a dermal oil of the Atlantic leatherback turtle. J. Am. Oil Chem. Soc. 42, 38–42 (1965)
Ackman, R. G., D. M. Nash and J. McLachlan: Lipids and fatty acids of Corophium volutator from Minas Basin. Proc. Trans. Nova Scotian Inst. Sci. 29, 501–516 (1979)
Amenta, J. S.: A rapid chemical method for quantification of lipids separated by thin-layer chromatography. J. Lipid Res. 5, 270–273 (1964)
Bell, M. V., C. M. F. Simpson and J. R. Sargent: (n-3) and (n-6) polyunsaturated fatty acids in the phosphoglycerides of saltsecreting epithelia from two marine fish species. Lipids 18, 720–726 (1983)
Bell, M. V., C. M. F. Simpson and J. R. Sargent: Fatty acid analyses of polyphosphoinositides from the gills of the cod Gadus morhua. Biochem. Soc. Trans. (In press)
Berridge, M. J. and R. F. Irvine: Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature, Lond. 312 315–321 (1984)
Bligh, E. G. and W. J. Dyer: A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959)
Borowsky, B.: Reproductive patterns of three intertidal salt-marsh gammaridean amphipods. Mar. Biol. 55, 327–334 (1980)
Calow, P.: Ecology, evolution and energetics: a study in metabolic adaptation. Adv. ecol. Res. 10, 1–52 (1977)
Christiansen, F. B., and T. M. Fenchel: Evolution of marine invertebrate reproductive patterns. Theor. Popul. Biol. 16, 267–282 (1979)
Christie, W. W.: Lipid analysis: isolation, separation, identification and structural analysis of lipids, 338 pp. Oxford: Pergamon Press 1973
Clarke, A.: Lipid class and fatty acid composition of Chorismus antarcticus (Pfeffer) (Crustacea: Decapoda) at South Georgia. J. exp. mar. Biol. Ecol. 28, 297–314 (1977)
Clarke, A.: On living in cold water: K-strategies in Antarctic benthos. Mar. Biol. 55, 111–119 (1979 a)
Clarke, A.: Lipid content and composition of the pink shrimp, Pandalus montagui (Leach) (Crustacea: Decapoda). J. exp. mar. Biol. Ecol. 38, 1–17 (1979 b)
Clarke, A.: Temperature and embryonic development in polar marine invertebrates. Int. J. Invertebrate Reprod. (Amsterdam) 5, 71–82 (1982)
Clarke, A.: Life in cold water: the physiological ecology of polar marine ectotherms. Oceanogr. mar. Biol. A. Rev. 21, 341–453 (1983)
Clarke, A.: The lipid content and composition of some Antarctic macrozooplankton. Br. Antarct. Surv. Bull. 63, 57–70 (1984 a)
Clarke, A.: Lipid composition of two species of Serolis (Crustacea, Isopoda) from Antarctica. Br. Antarct. Surv. Bull. 64, 37–53 (1984 b)
Fewster, M. E., B. J. Burns and J. F. Mead: Quantitative densitometric thin-layer chromatography using copper acetate reagent. J. Chromat. 43, 120–126 (1969)
Girard, J. P., A. J. Thomson and J. R. Sargent: Adrenalin induced turnover of phosphatidic acid and phosphatidylinositol in chloride cells from the gills of Anguilla anguilla. Fedn eur. biochem. Soc. (FEBS) Lett. 73, 267–270 (1977)
Gnaiger, E. and G. Bitterlich: Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia (Berl.) 62, 289–298 (1984)
Hartnoll, R. G. and S. M., Smith: Pair formation and the reproductive cycle in Gammarus duebeni. J. nat. Hist. 12, 501–511 (1978)
Johnson, S. B. and Y. G. Attramadal Reproductive behaviour and larval development of Tanais cavolinii, (Crustacea: Tanaidacea). Mar. Biol. 71, 11–16 (1982)
Kharoof, H. H.: Studies on the biochemical composition, metabolism and feeding efficiency of the amphipod Gammarus duebeni (Lilljeborg), 210 pp. Ph.D. thesis, University of Southampton, England 1970
Lawrence, J. M., J. B. McClintock and A. Guille: Organic level and caloric content of eggs of brooding asteroids and an echinoid (Echinodermata) from Kerguelen (South Indian Ocean) Int. J. Invertebrate Reprod. (Amsterdam) 7, 249–257 (1984)
McLaren, I. A., C. J. Corkett and E. J. Zillioux Temperature adaptations of copepod eggs from the arctic to the tropics. Biol. Bull. mar. biol. Lab., Woods Hole 137, 486–493 (1969)
Menge, B. A.: Brood or broadcast? The adaptive significance of different reproductive strategies in the two intertidal seastars Leptasterias hexactis and Pisaster ochraceus. Mar. Biol. 31, 87–100 (1975)
Moore, J. W.: The proximate and fatty acid composition of some estuarine crustaceans. Estuar. cstl mar. Sci. 4, 215–224 (1976)
Morris, R. J.: Relationships between the sex and degree of maturity of marine crustaceans and their lipid compositions J. mar. biol. Ass. U.K. 53, 27–37 (1973)
Morris, R. J.: The endemic faunae of Lake Baikal: their general biochemistry and detailed lipid composition. Proc. R. Soc. (Ser. B) 222 51–78 (1984)
Nair, K. K. C. and K. Anger: Seasonal variation in population structure and biochemical composition of Jassa falcata (Crustacea, Amphipoda) off the Island of Helgoland (North Sea). Estuar. cstl mar. Sci. 11, 505–513 (1980)
Nelson, W. G.: Reproductive patterns of gammaridean amphipods. Sarsia 65, 61–71 (1980)
Paradis, M. and R. G. Ackman: Localization of marine source of odd chain-length fatty acids. I. The amphipod Pontoporeia femorata (Krøyer). Lipids 11, 863–870 (1976)
Pearse, J. S. and A. C. Giese: The organic constitution of several benthonic invertebrates from McMurdo Sound, Antarctica. Comp. Biochem. Physiol. 18, 47–57 (1966)
Percy, J. A.: Seasonal changes in organic composition and caloric content of an arctic, marine amphipod, Onisimus (=Boeckosimus) affinis H. J. Hansen. J. exp. mar. Biol. Ecol. 40, 183–192 (1979)
Percy, J. A. and F. J. Fife. The biochemical composition and energy content of arctic marine macrozooplankton. Arctic 34, 307–313 (1981)
Perron, F. E. and R. H. Carrier: Egg size distributions among closely related marine invertebrate species: are they bimodal or unimodal? Am. Nat. 118, 749–755 (1981)
Rakusa-Suszczewski, S. and H. Dominas: Chemical composition of the Antarctic amphipod Paramoera walkeri Stebbing and chromatographic analysis of its lipids. Polskie Archwm Hydrobiol. 21, 261–268 (1974)
Richardson, M. G.: The ecology (including physiological aspects) of selected Antarctic marine invertebrates associated with inshore macrophytes, 191 pp. Ph.D. thesis, University of Durham 1977
Sheader, M.: The reproductive biology and ecology of Gammarus duebeni (Crustacea: Amphipoda) in southern England. J. mar. biol. Ass. U.K. 63, 517–540 (1983)
Skadsheim, A.: The ecology of intertidal amphipods in the Oslofjord. The life cycles of Chaetogammarus marinus and C. steoerensis. Pubbl. Staz. zool. Napoli (I. Mar. Ecol.) 3 213–224 (1982)
Skadsheim, A.: The ecology of intertidal amphipods in the Oslofjord. Distribution and responses to physical factors. Crustaceana 44, 225–244 (1983)
Skadsheim, A.: Life cycles of Gammarus oceanicus and G. salinus (Amphipoda), in the Oslofjord, Norway. Holarct. Ecol., Copenhagen 7, 262–270 (1984 a)
Skadsheim, A.: Coexistence and reproductive adaptations of amphipods: the role of environmental heterogeneity. Oikos 43, 94–103 (1984 b)
Stearns, S. C.: Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3–47 (1976)
Steele, D. H.: Correlation between egg size and developmental period. Am. Nat. 111, 371–372 (1977)
Steele, D. H. and V. J. Steele: The biology of Gammarus (Crustacea, Amphipoda) in the northwestern Atlantic. XI. Comparison and discussion. Can. J. Zool. 53, 1105–1109 (1975 a)
Steele, D. H. and V. J. Steele: Egg size and duration of embryonic development in Crustacea. Int. Revue ges. Hydrobiol. 60, 711–715 (1975 b)
Strathmann, R. R. and M. F. Strathmann: The relationship between adult size and brooding in marine invertebrates. Am. Nat. 119, 91–101 (1982)
Strathmann, R. R. and K. Vedder: Size and organic content of eggs of echinoderms and other invertebrates as related to developmental strategies and egg eating. Mar. Biol. 39, 305–309 (1977)
Thorson, G.: Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 25, 1–45 (1950)
Thurston, M. H.: The Crustacea Amphipoda of Signy Island, South Orkney Islands. Scient Rep. Br. Antarct. Surv. 71, 1–133 (1972)
Turner, P. B., M. Sheetz and L. A. Jaffe: Fertilisation increases the polyphosphoinositide content of sea urchin eggs. Nature, Lond. 310, 414–415 (1984)
Vader, W. J. M.: Amfipode-slektene Gammarus og Marinogammarus i Norge, med en illustrert bestemmelsestabell til de Nordvest-Europeiske arter (mimeograph) 23 pp. Blomsterdalen, Norway: Biologisk stasjon 1972
Van Dolah, R. F. and E. Bird: A comparison of reproductive patterns in epifaunal and infaunal gammaridean amphipods. Estuar. cstl mar. Sci. 11, 593–604 (1980)
Vance, R. R.: On reproductive strategies in marine benthic invertebrates. Am. Nat. 107, 33–352 (1973 a)
Vance, R. R.: More on reproductive strategies in marine benthic invertebrates. Am. Nat. 107, 353–361 (1973 b)
Whitaker, M. and R. F. Irvine: Inositol 1,4,5-triphosphate microinjection activates sea urchin eggs. Nature, Lond. 312, 636–639 (1984)
Author information
Authors and Affiliations
Additional information
Communicated by J. Mauchline, Oban
Rights and permissions
About this article
Cite this article
Clarke, A., Skadsheim, A. & Holmes, L.J. Lipid biochemistry and reproductive biology in two species of Gammaridae (Crustacea: Amphipoda). Marine Biology 88, 247–263 (1985). https://doi.org/10.1007/BF00392587
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392587