Summary
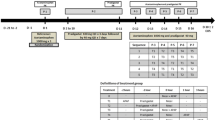
Procainamide was given to 20 patients with normal renal function as an i.v. bolus of 500 mg followed by 1.0 or 1.5 g eight-hourly by mouth in the form of a slow release preparation (Durules). 97.6±27.1 (SD)% of the oral procainamide was absorbed, the absorption half life being 1.54 h. The elimination half life following the oral formulation was 6.0±0.8 h, compared to a mean of 3.4±0.4 h following i.v. administration. Elimination half life following i.v. administration was slightly related to acetylator status, being 2.75±0.9 h in fast acetylators, and 4.4±2.4 h in slow acetylators. This dependence on acetylator status was not seen in half life following oral administration. Total body clearance, steady state plasma procainamide and N-acetylprocainamide were not significantly dependent on acetylator status, although a few patients who are slow acetylators had unexpectedly low clearance and high steady state procainamide concentrations when given the higher dose.
Similar content being viewed by others
References
Campbell, W., Tilstone, W. J., Lawson, D. H., Hutton, I., Lawrie T. D. V.: Acetylator phenotype and the clinical pharmacology of slow release procainamide. Br. J. clin. Pharmacol.3, 1023–1026 (1976)
Galeazzi, R. L., Benet, L. Z., Scheiner, L. B.: Relationship between the pharmacokinetics and pharmacodynamics of procainamide. Clin. Pharmacol. Ther.20, 278–289 (1976)
Gibaldi, M., Perrier, D.: Pharmacokinetics. New York: Marcel Dekker 1975
Giardina, E. G., Dreyfuss, J., Bigger, J. T., Shaw, J. M., Schreiber, E. C.: Metabolism of procainamide in normal and cardiac subjects. Clin. Pharmacol. Ther.19, 339–351 (1976)
Graffner, C., Johnsson, G., Sjogren, J.: Pharmacokinetics of procainamide intravenously and orally as conventional and slow release tablets. Clin. Pharmacol. Ther.17, 414–423 (1975)
Koch-Weser, J., Klein, S. W.: Procainamide dosage schedules, plasma concentrations and clinical effects. J. Am. med. Ass.215, 1454–1460 (1971)
Lawson, D. H., Jick, H.: Adverse reactions to procainamide. Br. J. clin. Pharmacol.4, 507–511 (1977)
Lima, J. J., Jusko, W. J.: Determination of procainamide acetylator status. Clin. Pharmacol. Ther.23, 25–29 (1978)
Price Evans, D. A.: An improved and simplified method of detecting the acetylator phenotype. J. Med. Genet.6, 405–407 (1969)
Simons, K. J., Levy, R. H., Cutler, R. E., Christopher, G. T., Lindner, A.: The pharmacokinetics of procainamide in normal subjects using a specific gas chromatographic assay. Res. Commun. chem. Pathol. Pharmacol.11, 173–185 (1975)
Tilstone, W. J., Lawson, D. H.: Capacity-limited elimination of procainamide in man. Res. Commun. chem. Pathol. Pharmacol.21, 343–346 (1978)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tilstone, W.J., Lawson, D.H., Campbell, W. et al. The pharmacokinetics of slow-release procainamide. Eur J Clin Pharmacol 14, 261–265 (1978). https://doi.org/10.1007/BF00560459
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00560459