Summary
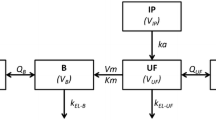
We have fitted a first-order multicompartment pharmacokinetic model to plasma platinum concentrations measured in nine ovarian cancer patients who received intravenous infusions of cisplatin for 6 h.
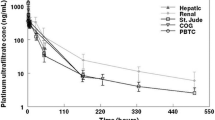
The time-course of ultrafilterable plasma platinum was similar in all patients studied, and was fitted by a single compartment within the limits of experimental detection. However, the time-course of protein-bound platinum showed marked differences between patients, the differences being explained by distribution to two peripheral compartments.
The wide inter-patient variation observed in protein-bound plasma platinum concentrations supports the view that pharmacokinetic modelling should be carried out separately for each patient, since averaging plasma concentrations would have obscured some individual pharmacokinetic characteristics.
Similar content being viewed by others
References
Himmelstein KJ, Patton TF, Belt RJ, Tayler S, Repta AJ, Sternson LA (1981) Clinical kinetics of intact cisplatin and some related species. Clin Pharmacol Ther 29: 658–664
Evans WE, Crom WR, Tsiatis A, Green AA, Hayes FA, Pratt CB (1982) Pharmacokinetic modelling of cisplatin disposition in children and adolescents with cancer. Cancer Chemother Pharmacol 10: 622–626
Vermorken JB, van der Vigh WJF, Klein I, Hart AAM, Gall HE, Pinedo HM (1984) Pharmacokinetics of free and total platinum species after short-term infusions of cisplatin. Cancer Treat Rep 68: 505–513
Fish RG, Shelley MD, Griffiths H (1984) Total body clearance and platinum accumulation in patients treated with cis-dichlorodiammine platinum (II). Ther Drug Monit 6: 251–252
Martin E, Moll W, Schmid P, Dettli L (1984) Problems and pitfalls in estimating average pharmacokinetic parameters. Eur J Clin Pharmacol 26: 595–602
Shelley MD, Fish RG (1985) Platinum analysis in biological fluids by graphite furnace atomic absorption spectrophotometry. Presented at Assoc Clin Biochem, Strategy '85, Cardiff
Gibaldi M, Perrier D (1982) Multicompartment models. In: Pharmacokinetics, 2nd ed. Marcel Dekker Inc, New York Basel
Buice RG, Soloway MS (1980) Potential effects of edema on the pharmacokinetics of platinum in patients treated with cis-dichlorodiammine platinum (II). Res Commun Chem Pathol Pharmacol 30: 447–457
Gullo JJ, Litterst CL, Maguire PJ, Sidik BI, Hoth DF, Wooley PV (1980) Pharmacokinetics and protein binding of cis-dichlorodiammine platinum (II) administered as a one hour or a twenty hour infusion. Cancer Chemother Pharmacol 5: 21–26
Shelley MD, Frayling L, Fish RG (1986) Plasma protein binding of platinum complexes in vitro. Presented at Assoc Clin Biochem, Focus '86, Glasgow
Cooper EH, Stone J (1979) Acute phase reactant proteins in cancer. In: Klein G, Weinhouse I (eds) Advances in cancer research, vol 30. Academic Press, London, pp 1–44
Schoenberger JA, Kroll G, Sakamoto A, Kark RM (1952) Investigation of the permeability factor in ascites and edema using albumin tagged with 125I. Gastroenterology 22: 607–622
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Griffiths, H., Shelley, M.D. & Fish, R.G. A modified pharmacokinetic model for platinum disposition in ovarian cancer patients receiving cisplatin. Eur J Clin Pharmacol 33, 67–72 (1987). https://doi.org/10.1007/BF00610382
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610382