Summary
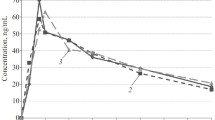
Healthy male volunteers received three different dose regimens of a controlled-release form of isosorbide-5-mononitrate (IS-5-MN; 60 mg per tablet). Dose regimen I consisted of a single daily dose of 60 mg given for 5 days. Dose regimen 11 was started with a dose of 60 mg, followed by 30 mg 12 h later and thereafter every 8 h. The last dose, on the 5th day was again 60 mg. In dose regimen III60 mg followed by 30 mg 6 h later were administered every day for 5 days. The peripheral arterial and venous effects of IS-5-MN during the first and last dosing interval were followed by changes in the finger pulse curve, standing systolic blood pressure, heart rate, and venous distensibility. Plasma concentrations of IS-5-MN were measured frequently following the first and the last dose. Following dose regimen I all hemodynamic effects produced by the first dose were maintained during the study. The maximal plasma concentrations were about 400 ng/ml and the trough value, lower than 100 ng/ml. Following dose regimen II the hemodynamic effects of IS-5-MN and sublingual glyceroltrinitrate were completely abolished on the 5th day. Trough plasma concentrations were approximately 300 ng/ml during the entire study period. Following dose regimen III pronounced hemodynamic effects were seen on the 1st day. However, a significant attenuation of the hemodynamic effects was measured on the 5th day, when trough plasma concentrations were between 100 and 230 ng/ml. There was a significant negative correlation between the magnitude of hemodynamic effect remaining on the 5th day (measured by the area under the finger pulse curve) and the trough plasma concentration. Thus, the maintenance of minimum plasma concentrations of IS-5MN of 300 ng/ml or higher produces a rapid development of hemodynamic nitrate tolerance, whereas no tolerance was found when the plasma concentrations were allowed to decline below 100 ng/ml before the next dose was given. A significant attenuation of hemodynamic effects was found when minimum plasma concentrations were between 100 and 230 ng/ml. The degree of attenuation in this concentration range increased with increasing trough plasma concentrations.
Similar content being viewed by others
References
Thadani U, Fung HL, Darke AC, Parker JO (1982) Oral isosorbide dinitrate in angina pectoris: comparison of duration of action and dose-response relation during acute and sustained therapy. Am J Cardiol 49: 411–419
Parker JO, Fung HL, Rugirello D, Stone JA (1983) Tolerance to isosorbide dinitrate: rate of development and reversal. Circulation 68: 1074–1080
Abrams J (1986) Tolerance to organic nitrates. Circulation 74: 1181–1185
Rudolph W, Blasini R, Reiniger G, Brügmann U (1983) Tolerance development during isosorbide dinitrate treatment: can it be circumvented? Z Kardiol 72 [Suppl 3]: 195–198
Silber S, Krause K-H, Theisen K (1984) Nitrate tolerance: Dependence on dosage intervals? Circulation 70 [Suppl II]: II-189 (Abstract 753)
Down WH, Chasseaud LF, Grundy RK (1974) Biotransformation of isosorbide dinitrate in humans. J Pharm Sci 63: 1147–1149
Parker JO (1987) Nitrate therapy in stable angina pectoris. N Engl J Med 316: 1635–1642
Ignarro LJ, Lippton H, Edwards JC et al (1981) Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther 218: 739–749
Abshagen U, Betzien G, Endele R, Kaufmann B (1981) Pharmacokinetics of intravenous and oral isosorbide-5-mononitrate. Eur J Clin Pharmacol 20: 269–275
Schinz A, Gottsauner A, Schnelle K (1981) Digital pulse plethysmography: a sensitive test of the pharmacodynamics of nitratesreproducibility and quantitation of the technique. In: Lichtlen PR, Engel HJ, Schrey A, Swan HJC (eds) Nitrates III. Cardiovascular effects. Springer, Berlin Heidelberg New York, pp 117–122
Hellige G, Ensink FB, Baller D, Prennschütz-Schützenan H, Sigmund-Duchanova H, Zipfel J (1979) Measurement of arterial and venous reactivity by an advanced straingauge plethysmograph. Angiology 30: 539–549
Ahnoff M, Holm G (1981) Automated gas chromatographic determination of isosorbide dinitrate, isosorbide-2-mononitrate and isosorbide-5-mononitrate in plasma. In: Kaiser RE (ed) Proceedings of the 4th International Symposium on Capillary Chromatography. Hüthig, Heidelberg Basel New York, pp 673–686
Morikawa Y (1967) Characteristic pulse wave caused by organic nitrates. Nature 213: 841–842
Imhoff PR, Ott B, Frankhauser P, Chu L-C, Hodler J (1980) Difference in nitroglycerin dose-response in the venous and arterial beds. Eur J Clin Pharmacol 18: 455–460
Wille HH, Sauer G, Tebbe U, Neuhaus KL, Kreuzer H (1980) Nitroglycerin and afterload: effects of aortic compliance and capacity of theWindkessel. Eur Heart J 1: 445–452
Tauchert M, Jansen W, Osterspey A (1985) Nitrate, Nitratdosen und Toleranz. In: Borchard U, Rafflenbeul W, Schrey A (eds) Mononitrat. Wolf, Munich, pp 45–46
Fung H-L, Sutton SC, Kamiya A (1984) Blood vessel uptake and metabolism of organic nitrates in the rat. J Pharmacol Exp Ther 228: 334–341
Silber S (1984) Nitrattoleranz: pro und contra. Dtsch Med Wochenschr 109: 1124–1132
Kaltenbach M, Schneider W (1986) Fortbestehen der antianginösen Wirksamkeit unter chronischer Nitrattherapie trotz Aufhebung hämodynamischer Teileffekte. Dtsch Med Wochenschr 111: 383–386
Boertz A, Bonn R (1986) Nitrate therapy without loss of action by correct dosage. Z Kardiol 75 [Suppl 3]: 57
Parker JO, Farrell B, Lahey KA, Moe G (1987) Effect of intervals between doses on the development of tolerance to isosorbide dinitrate. N Engl J Med 316: 1440–1444
Blasini R, Brügmann U, Reiniger G, Rudolph W (1985) Longterm treatment of exercise-induced angina pectoris with oncedaily administration of 120 mg isosorbide dinitrate in sustainedrelease form. Herz 10: 163–171
Ohlmeier H, Mertens HM, Möller M, Mannebach H, Gleichmann U (1986) Vereinfachte Langzeittherapie der koronaren Herzkrankheit mit 120 mg retardiertem Isosorbiddinitrat einmal täglich. Untersuchung zur Wirkdauer und Toleranzentwicklung. Z Kardiol 75 [Suppl 3]: 50–56
Silber S, Vogler AC, Krause K-H, Vogel M, Theisen K (1987) Induction and circumvention of nitrate tolerance applying different dosage intervals. Am J Med 83: 860–870
Thadani U, Hamilton SF, Olson E et al (1987) Duration of effects and tolerance of slow-release isosorbide-5-mononitrate for angina pectoris. Am J Cardiol 59: 756–762
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wagner, F., Siefert, F., Trenk, D. et al. Relationship between pharmacokinetics and hemodynamic tolerance to isosorbide-5-mononitrate. Eur J Clin Pharmacol 38 (Suppl 1), S53–S59 (1990). https://doi.org/10.1007/BF01417565
Issue Date:
DOI: https://doi.org/10.1007/BF01417565