Summary
A randomised, single-blind comparative study was carried out in 9 ovariectomized women to evaluate the kinetics of single doses of three different steroid combinations: 0.150 mg desogestrel +2.0 mg micronized 17β-oestradiol, 0.150 mg desogestrel +0.500 mg 17β-oestradiol cyclo-octyl acetate and 0.150 mg desogestrel +1.0 mg 17β-oestradiol decanoate. Serum levels of 17β-oestradiol and oestrone were measured, as well as the excretion of 17β-oestradiol and its metabolites (oestrone and oestriol) in urine.
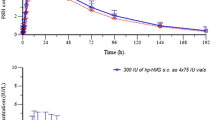
In relation to the doses given, higher peak serum concentrations of 17β-oestradiol were obtained after the two fat soluble analogues, while the AUCs were similar to that after micronised 17β-oestradiol. However, there was more extensive conversion of the micronised 17β-oestradiol preparation into oestrone compared to 17β-oestradiol cyclo-octyl acetate and 17β-oestradiol decanoate. The oestrone/17β-oestradiol serum concentration ratio was approximately 2.6 before tablet intake and remained essentially unchanged after intake of 17β-oestradiol cyclo-octyl acetate and 17β-oestradiol decanoate. After micronized 17β-oestradiol however, there was a 2–3-fold increase in the ratio at Cmax and slower elimination of 17β-oestradiol from plasma, which may be due to the fact that high serum oestrone levels may serve as a reservoir, since both a metabolite and also a precursor of 17β-oestradiol.
The urinary excretion of 17β-oestradiol, oestrone and oestriol was highest after oral administration of micronized 17β-oestradiol compared to 17β-oestradiol cyclo-octyl acetate and 17β-oestradiol decanoate. The time pattern of urinary excretion reflected the serum concentration profiles of the preparations given.
Similar content being viewed by others
References
Åstedt B, Jeppson S (1974) 17β-oestradiol and the fibrinolytic activity of vein walls. J Obstet Gynaecol Br Cwlth 81: 81–719
Kicovic PM, Luisi M, Franchi F, Alicicco E (1977) Effects of orally administered oestradiol decanoate on plasma oestradiol, oestrone and gonadotropin levels, vaginal cytology, cervical mucus and endometrium in ovariectomized women. Clin Endocrinol 7: 73–77
Luisi M, Kicovic PM, Alicicco E, Franchi F (1978) Effects of oestradiol decanoate in ovariectomized women. J Endocrin Invest 2: 101–106
Schubert W, Cullberg G (1988) Fat-soluble 17β-oestradiol: a way of reducing dosage in steroid hormonal substitution? Acta Obstet Gynaecol Scand 67: 271–275
Dahlgren E, Crona N, Jansson PO, Samsioe G (1985) Oral replacement with oestradiol cyclooctyl acetate: a new oestradiol analogue. Gynaecol Obstet Invest 20: 84–90
Holst J (1983) Percutaneous oestrogen therapy: endometrial response and metabolic effect. Acta Obstet Gynaecol Scand Suppl 115: 1–30
Steingold K, Stumpf P, Kresner D, Liu H-C, Narof D, Rosenwaks Z (1989) Estradiol and progesterone replacement regimens for the induction of endometrial eceptivity. Fertil Steril 52: 756–760
Cullberg G (1985) Pharmacodynamic studies on desogestrel administered alone and in combination with ethinyl oestradiol. Acta Obstet Gynaecol Scand Suppl 133: 1–29
Schubert W, Cullberg G (1987) Ovulation inhibition with 17β-oestradiol cyclo-octyl acetate and desogestrel. Acta Obstet Gynaecol Scand 66: 543–547
Metropolitan Life Insurance Company (1960) Frequence of overweight and underweight. Stat Bull Metrop Life Insur Co 41: 4–10
Gibaldi M, Perrier D (1982) Pharmacokinetics. Dekker, New York, pp 33–40
Young RL, Goldzieher JW (1987) Current status of postmenopausal oestrogen therapy. Drug 33: 95–106
de Visser J, van der Vies J (1977) Oestrogenic activity of oestradiol-decanoate after oral administration to rodents. Acta Endocrinologica 85: 422–428
Hershcopf J, Bradlow HL, Fishman J, Swanec GE, Larner JM, Hochberg RB (1985) Metabolism of oestadiol fatty acid esters in man. J Clin Endocrinol Metabol 61: 1071–1075
Larner JM, Rosner W, Hochberg RB (1987) Binding of oestradiol-17-fatty acid esters to plasma proteins. Endocrinology 121: 738–744
Kuhl H (1990) Pharmacokinetics of oestrogens and progestogens. Maturitas 12: 171–197
Hümpel M, Nieuweboer B, Wendt H, Speck U (1979) Investigatons of pharmacokinetics of ethinyloestradiol to specific consideration of a possible first-pass effect in women. Contraception 19: 421–432
Horst HJ, Höltje WJ, Dennis M, Coert A, Geelen J, Voigt KD (1976) Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr 54: 875–879
Lyrenäs S, Carlström K, Bäckström T, von Schoultz B (1981) A comparison of serum oestrogen levels after percutaneous and oral administration of oestradiol-17β. Br J Obstet Gynaecol 88: 181–187
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schubert, W., Cullberg, G. & Hedner, T. Pharmacokinetic evaluation of oral 17β-oestradiol and two different fat soluble analogues in ovariectomized women. Eur J Clin Pharmacol 44, 563–568 (1993). https://doi.org/10.1007/BF02440860
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02440860