Abstract
Objective: To determine population pharmacokinetic parameters of caffeine in premature neonates.
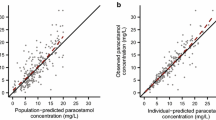
Methods: This population analysis was done using 145 serum concentration measurements gathered from 75 hospitalized patients during their routine clinical care. The data were analysed by use of NONMEM (mixed effects modelling) according to a one-compartment open model with either zero or first-order absorption and first-order elimination. The effect of a variety of developmental, demographic and clinical factors (gender, birth weight, current weight, gestational age, postnatal age, postconceptional age and concurrent treatment with phenobarbital and parenteral nutrition) on clearance and volume of distribution was investigated. Forward selection and backward elimination regression identified significant covariates.
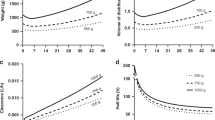
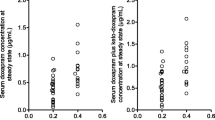
Results: The final pharmacostatistical model with influential covariates were as follows: clearance (ml · h−1) =5.81 · current weight (kg) + 1.22 · postnatal age (weeks), multiplied by 0.757 if gestational age ≤ 28 weeks and 0.836 if the current primary source of patients' nutrition is parenteral nutrition, and volume of distribution (ml) = 911 · current weight (kg). The interindividual variability in clearance and the residual variability, expressed as coefficients of variation, were 14.87% and 18.44%, respectively. Due to the lack of information on the data set we were unable to characterize the interindividual variability for volume of distribution.
Conclusion: In this study, which involved on average only two serum concentrations of caffeine per patient, the use of NONMEM gave us significant and consistent information about the pharmacokinetic profile of caffeine when compared with available bibliographic information. Additionally, parenteral nutrition and low gestational age (≤ 28 weeks) may even come to be considered as risk factors, and their presence may serve as an indicator of the need for periodic monitoring of caffeine concentrations in premature infants.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 July 1996 / Accepted in revised form: 26 November 1996
Rights and permissions
About this article
Cite this article
Falcão, A., de Gatta, M., Iribarnegaray, M. et al. Population pharmacokinetics of caffeine in premature neonates. E J Clin Pharmacol 52, 211–217 (1997). https://doi.org/10.1007/s002280050276
Issue Date:
DOI: https://doi.org/10.1007/s002280050276