Summary
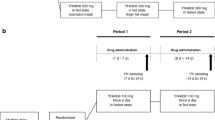
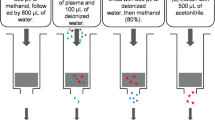
Single doses of β-methyl-digoxin 0.4 mg were given to groups of 17 – 18 healthy volunteers as an intravenous infusion lasting 2 hours, or orally as Lanitop Liquidum® or Lanitop® tablets. The serum glycoside concentration and urinary glycoside excretion were measured over 8 and 32 h. The absolute bioavailability from the oral preparations in comparison with the infusion was lower for the first 8 h than for the entire 32 h of the investigation; the relative bioavailability from tablets was the same as from the solution for both periods. For both periods the area under the serum concentration/time curve and the urinary glycoside excretion were significantly lower after administration of the tablets than after intravenous infusion. Taking the average of both parameters, the absolute bioavailability of β-methyl-digoxin was about 80% from the solution and about 70% from the tablets. In 18 patients undergoing intravenous or oral therapy with β-methyl-digoxin steady state glycoside concentrations were compared in a cross-over study of intravenous maintenance therapy with Lanitop® ampoules or oral treatment with Lanitop® tablets. For a standard daily dose of 0.2 mg β-methyl-digoxin the serum concentrations were 1.35±0.10 ng/ml during both intravenous and oral administration. The intra-individual variation in glycoside concentration after changing from intravenous to oral maintenance therapy, or vice versa, was about the same as during continued intravenous or oral administration. It is concluded that the rate of rise of serum concentration after a single dose may be a useful indicator of the rate of absorption, but that the area under the serum concentration/time curve and the urinary glycoside excretion up to 32 h are unsuitable for determining equivalent doses of different formulations or routes of administration of digitalis glycosides.
Similar content being viewed by others
References
Belz, G.G., Kleeberg, U.R.: Plasma half life of β-methyl-digoxin following repetitive application in man. Klin. Wschr.53, 491–492 (1975)
Beveridge, T., Kalberer, F., Nüesch, E., Schmidt, R.: Bioavailability studies with Digoxin-Sandoz® and Lanoxin®. Europ. J. clin. Pharmacol.8, 371–376 (1975)
Doering, W., König, E., Kronski, D., Hall, D.: Bestimmung der Kenngrößen von β-Methyldigoxin mit Einschwemmkatheterverfahren und nicht-invasiven Methoden. Dtsch. med. Wschr.98, 2274–2280 (1973)
Fleckenstein, L., Weintraub, M., Kroenig, B., Lasagna, L.: Assessment of the biologic availability of digoxin preparations in man. Clin. Pharmacol. Ther.15, 205 (1974)
Haasis, R., Larbig, D., Klenk, K.: Glykosidkonzentration im Serum und Urin bei Herzgesunden nach Gabe von Beta-Methyl-Digoxin. Klin. Wschr.53, 529–533 (1975)
Härtel, G., Manninen, V., Melin, J., Apajalahti, A.: Serum-digoxin concentrations with a new digoxin derivative, β-methyl-digoxin. Ann. clin. Res.5, 87–90 (1973)
Huffmann, D.H., Manion, C.V., Azarnoff, D.L.: Absorption of digoxin from different oral preparations in normal subjects during steady state. Clin. Pharmacol. Ther.16, 310–317 (1974)
König, E., Ohly, A.: Quantitative Eigenschaften eines neuen Herzglykosids. Med. Klin.65, 296–299 (1970)
Larbig, D., Kochsiek, K.: Radioimmunchemische Bestimmung von Digoxin im menschlichen Serum. Klin. Wschr.49, 1031–1032 (1971)
Larbig, D., Scheler, F., Schmidt, H.-J., Betzien, G., Kaufmann, B.: Untersuchungen zur enteralen Resorption von β-Methyldigoxin. Klin. Wschr.49, 604–607 (1971)
Lindenbaum, J.: Bioavailability of different lots of digoxin tablets from the same manufacturer. Clin. Pharmacol. Ther.17, 296–301 (1975)
Lindenbaum, J.: Bioavailability of digoxin tablets. Pharmacol. Rev.25, 229–237 (1973)
Ochs, H., Bodem, G., Hahn, E., Dengler, H.J.: Vergleichende Untersuchungen der biologischen Verfügbarkeit von Digoxin aus Tabletten und Lösung im Langzeitversuch. Klin. Wschr.53, 425–429 (1975)
Preibisz, J.J., Butler, V.P., Lindenbaum, J.: Digoxin tablet bioavailability: Single-dose and steady-state assessment. Ann. intern. Med.81, 469–474 (1974)
Redfors, A.: Plasma digoxin concentration — its relation to digoxin dosage and clinical effects in patients with atrial fibrillation. Brit. Heart J.34, 383–391 (1972)
Rietbrock, N., Guggenmos, J.: Bioavailability of digoxin and derivatives. Naunyn-Schmiedeberg's Arch. Pharmacol.282, R 80 (1974)
Rietbrock, N., Guggenmos, J., Kuhlmann, J., Hess, U.: Bioavailability and pharmacokinetics of β-methyldigoxin after multiple oral and intravenous doses. Europ. J. clin. Pharmacol. in press 1976
Sanchez, N., Sheiner, L.B., Halkin, H., Melmon, K.L.: Pharmacokinetics of digoxin: Interpreting bioavailability. Brit. med. J.4, 132–134 (1973)
Schaumann, W., Zielske, F., Kohler, K., Koch, K.: β-Methyl-digoxin: III. Speed of absorption in guinea-pigs in comparison to other cardiac glycosides. Naunyn-Schmiedeberg's Arch. Pharmacol.272, 32–45 (1972)
Schröder, G., Abrahamsson, L., Wassen, A., Malmcrona, R., Bergqvist, N.: Plasma concentration of digoxin in out-patients. Acta med. scand.193, 215–218 (1973)
Smith, T.W., Butler, V.P., Haber, E.: Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. New. Engl. J. Med.281, 1212–1216 (1969)
Steinorth, G., Schweers, A.: Bericht über die klinische Prüfung von Lanitop. Med. Welt24, 1310–1317 (1973)
Storz, H.: Die quantitative Wirksamkeit des Herzglykosids β-Methyldigoxin. Med. Welt21, 2066–2070 (1970)
Wagner, J.G.: Appraisal of digoxin bioavailability and pharmacokinetics in relation to cardiac therapy. Amer. Heart J.88, 133–138 (1974)
Wagner, J.G., Christensen, M., Sakmar, E., Blair, D., Yates, J.D., Willis, P.W., Sedman, A.J., and Stoll, R.G.: Equivalence lack in digoxin plasma levels. J. Amer. med. Ass.224, 199–204 (1973)
Weiss, W., Olcay, A., Teufel, W., Glocke, M.: Vergleichende Untersuchungen der Glykosid-Konzentration im Serum bei Erhaltungs-therapie mit Lanicor®, Card-Lamuran®, MF 708 d und Lanitop®. Med. Klinik70, 1367–1374 (1975)
White, R.J., Chamberlain, D.A., Howard, M., Smith, T.W.: Plasma Concentrations of digoxin after oral administration in the fasting and postprandial state. Brit. med. J.1, 380–381 (1971)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boerner, D., Olcay, A., Schaumann, W. et al. Absorption of β-methyl-digoxin determined after a single dose and under steady state conditions. Eur J Clin Pharmacol 9, 307–314 (1976). https://doi.org/10.1007/BF00561665
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00561665