Summary
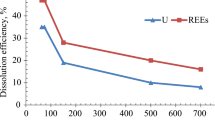
The solubilization of phosphate was investigated using apatite and P-containing uranium rock samples in acid solutions. The concentration of soluble phosphate usually increased upon 24-h extraction followed by subsequent precipitation. Uranium solubilization was investigated using leach solutions that contained about 3 g/l ferric iron produced by bacterial oxidation of iron pyrite. 1–94% extraction of uranium was attained within 24h, with the poor recovery associated with a rock sample containing an excessive amount of alkaline material. Reducing equivalents (permanganate-consuming substances) in the acid leach solutions could be oxidized by inoculation with iron-oxidizing thiobacilli (Thiobacillus ferrooxidans). Chemical analysis of iron indicated that most of the iron was in the oxidized form. Stable iron colloids were not present in significant amounts in the leach solutions. Acidic ferric sulfate containing about 6 g/l Fe3+ was also used as a leach solution. Repeated use of the leach solution with rock samples did not decrease the efficiency of the leaching but soluble uranium reached a toxic concentration and thus prevented the bacterial re-oxidation of ferrous iron in the leach solution.
Similar content being viewed by others
References
Äikäs O (1980) Uraniferous phosphorite and apatite-bearing gneisses in the Proterozoic of Finland. In: Ferguson J, Goleby AB (eds) Uranium in the Pine Creek Geosyncline. International Atomic Energy Agency, Vienna, pp 675–681
Arkesteyn GJMW (1980) Pyrite oxidation in acid sulphate soils: the role of microorganisms. Plant Soil 54:119–134
Brierley JA (1978) Thermophilic iron-oxidizing bacteria found in copper leaching dumps. Appl Environ Microbiol 36:523–525
Brierley JA, Lockwood SJ (1977) The occurrence of thermophilic iron-oxidizing bacteria in a copper leaching system. FEMS Microbiol Lett 2:163–165
Brierley JA, Norris PR, Kelly DP, Le Roux NW (1978) Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. Eur J Appl Microbiol Biotechnol 5:291–299
Guay R, Silver M (1981) Uranium biohydrometallurgy. Process Biochem 15:8–11, 44
Guay R, Silver M, Torma AE (1977) Ferrous iron oxidation and uranium extraction by Thiobacillus ferrooxidans. Biotechnol Bioeng 14:727–740
Heaney SI, Davison W (1977) The determination of ferrous iron in natural waters with 2,2′-bipyridyl. Limnol Oceanogr 22:753–760
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 5B. Academic Press, London, pp 209–344
Hiltunen P, Vuorinen A, Rehtijärvi P, Tuovinen OH (1981) Bacterial pyrite oxidation: release of iron and scanning electron microscopic observations. Hydrometallurgy 7:147–157
Irwing H, Mellor DH (1962) The stability of metal complexes of 1,10-phenanthroline and its analogues. Part 1. 1,10-phenanthroline and 2,2′-bipyridyl. J Chem Soc:5222–5237
Manchee RJ (1977) Laboratory-scale bacterially assisted leaching of Canadian uranium ores. Trans Instn Min Metall 86:C126-C133
Rehtijärvi P, Äikäs O, Mäkelä M (1979) A middle Precambrian uranium and apatite-bearing horizon associated with the Vihanti zinc ore deposit, Western Finland. Econ Geol 74:1102–1117
Rosenberg RJ (1981) A simple method for the determination of uranium and thorium by delayed neutron counting. J Radioanal Chem 62:145–149
Soljanto P, Rehtijärvi P, Tuovinen OH (1980) Ferrous iron oxidation by Thiobacillus ferrooxidans: inhibition by finely ground particles. Geomicrobiol J 2:1–12
Vaasjoki M, Äikäs O, Rehtijärvi P (1980) The age of mid-Proterozoic phosphate metasediments in Finland as indicated by radiometric U-Pb dates. Lithos 13:257–262
Vuorinen A, Hiltunen P, Hsu JC, Tuovinen OH (1983) Solubilization and speciation of iron during pyrite oxidation by Thiobacillus ferrooxidans. Geomicrobiol J (in press)
Wakao N, Mishina M, Sakurai Y, Shiota H (1982) Bacterial pyrite oxidation I. The effect of pure and mixed cultures of Thiobacillus ferrooxidans and Thiobacillus thiooxidans on release of iron. J Gen Appl Microbiol 28:331–343
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tuovinen, O.H., Hiltunen, P. & Vuorinen, A. Solubilization of phosphate, uranium, and iron from apatite- and uranium-containing rock samples in synthetic and microbiologically produced acid leach solutions. European J. Appl. Microbiol. Biotechnol. 17, 327–333 (1983). https://doi.org/10.1007/BF00499498
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00499498