Abstract
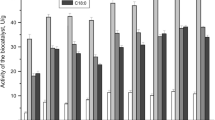
A thermostable β-galactosidase (EC 3.2.1.23) from a thermophilic anaerobe, strain NA10, was purified from the crude extract of the Escherichia coli transformant harboring the lacN gene. The purified enzyme was physically and covalently immobilized to a porous ceramic support, SM-10. Among the supports tested, the highest residual activity after 3 h incubation at 70° C was obtained when the enzyme was covalently immobilized to silanized SM-10 with 3-[2-(2-amino-ethylaminoethylamino)propyl]trimethoxysilane. The amount of the enzyme immobilized was about 60 mg/g of this support. The enzymatic properties were almost the same as those of the free enzyme. The half-life of this immobilized enzyme was estimated to be approximately 450 h at the pasteurization temperature (65° C).
Similar content being viewed by others
References
Banerjee M, Chakravarty A, Majumdar SK (1984) Characteristics of yeast β-galactosidase immobilized on calcium alginate gels. Appl Microbiol Biotechnol 20:271–274
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254
Kobayashi T, Hirose Y, Ohmiya K, Shimizu S, Uchino F (1978) Thermostable β-galactosidase from Bacillus acidocaldarius and its immobilization 56:309–314
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mozaffar Z, Nakanisi K, Matsuno R (1986) Continuous production of galacto-oligosaccharides from lactose using immobilized β-galactosidase from Bacillus circulans. Appl Microbiol Biotechnol 25:224–228
Saito T, Honda H, Iijima S, Kobayashi T (1989) Isolation of β-galactosidase gene from a thermophilic anaerobe and its expression in Escherichia coli. Enzyme Microb Technol 11:302–305
Saito T, Kato K, Suzuki T, Iijima S, Kobayashi T (1992a) Nucleotide sequence of the lacN gene encoding thermostable β-galactosidase of a thermophilic anaerobe, strain NA10. J Ferment Bioeng 73:51–53
Saito T, Kato K, Maeda S, Suzuki T, Shiba S, Iijima S, Kobayashi T (1992b) Overproduction of thermostable β-galactosidase in Escherichia coli, its purification and molecular structure. J Ferment Bioeng 74:12–16
Sissons CH, Sharrock KR, Daniel RM, Morgan HW (1987) Isolation of cellulolytic anaerobic extreme thermophiles from New Zealand thermal sites. Appl Environ Microbiol 53:832–838
Taya M, Hinoki H, Yagi T, Kobayashi T (1988) Isolation and characterization of an extremely thermophilic, cellulolytic anaerobic bacterium. Appl Microbiol Biotechnol 29:474–479
Woychik JH, Wondolowski MV (1973) Lactose hydrolysis in milk and milk products by bound fungal β-galactosidase. J Milk Food Technol 36:31–33
Yoshida Y, Kawase M, Majima T, Shiraisi T (1990) A bioreactor with immobilized enzyme on porous ceramics. Hakkoukougakukaishi 68:267–273
Author information
Authors and Affiliations
Additional information
On leave from Aichi Institute of Technology, Yakusa-cho, Toyota 470-03, Japan
Correspondence to: T. Saito
Rights and permissions
About this article
Cite this article
Saito, T., Yoshida, Y., Kawashima, K. et al. Immobilization and characterization of a thermostable β-galactosidase from a thermophilic anaerobe on a porous ceramic support. Appl Microbiol Biotechnol 40, 618–621 (1994). https://doi.org/10.1007/BF00173317
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173317