Abstract
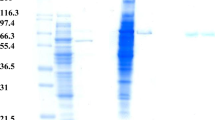
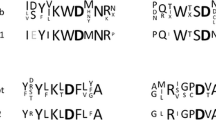
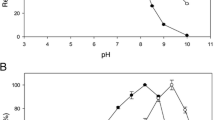
We purified an extracellular thermostable β-galactosidase of Saccharopolyspora rectivirgula strain V2-2, a thermophilic actinomycete, to homogeneity and characterized it to be a monomeric enzyme with a relative molecular mass of 145 000 and s°20,w of 7.1 s. In addition to the hydrolytic activity of 1-O-substituted β-d-galactopyranosides such as lactose [a Michaelis constant K m=0.75 mm and molecular activity (k cat)= 63.1 s−1 at pH 7.2 and 55° C] and p-nitrophenyl β-d-galactopyranoside (K m=0.04 mm k cat= 55.8 s−1), the enzyme had a high transgalactosylation activity. The enzyme reacted with 1.75 m lactose at 70°C and pH 7.0 for 22 h to yield oligosaccharides in a maximum yield (other than lactose) of 41% (w/w). A general structure for the major transgalactosylic products could be expressed as (Gal)c-Glc, where n is 1, 2, 3, and 4 with a glucose at a reducing terminal. These oligosaccharides could selectively promote the growth of the genus Bifidobacterium found in human intestines. S. rectivirgula β-galactosidase was stable at pH 7.2 up to 60°C (for 4 h in the presence of 10 μm MnCl2) or 70°C (for 22 h in the presence of 1.75 m lactose and 10 μm MnCl2). Thus the enzyme is applicable to an immobilized enzyme system at high temperatures (60°C <) for efficient production of the oligosaccharides from lactose.
Similar content being viewed by others
References
Davis BJ (1964) Disc electrophoresis — II. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Dickson RC, Dickson LR, Markin JS (1979) Purification and properties of an inducible β-galactosidase isolated from the yeast Kluyveromyces lactis. J Bacteriol 37:51–61
Fowler AV, Zabin I (1978) Amino acid sequence of β-galactosidase. XI. Peptide ordering procedures and the complete sequence. J Biol Chem 253:5521–5525
Gekas V, López-Leiva M (1985) Hydrolysis of lactose: a literature review. Process Biochem 2:2–12
Gerlach U (1983) Sorbitol dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 3, 3rd edn. Verlag Chemie. Weinheim, p 112
Greenberg NA, Mahoney RR (1981) Immobilization of lactase (β-galactosidase) for use in dairy processing: a review. Process Biochem 2:2–8
György P, Norris RF, Rose CS (1954) Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys 48:193–201
Kato Y, Komiya K, Sasaki H, Hashimoto T (1980) Comparison of TSK-gel PW type and SW type in high-speed aqueous gel-permeation chromatography. J Chromatogr 193:311–315
Kiel RA, Tanzer JM, Woodiel FN (1977) Identification, separation, and preliminary characterization of invertase and β-galactosidase in Actinomyces viscosus. Infect Immun 16:81–87
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Matsumoto K, Kuroda A (1988) Galactooligosaccharides. In: The amylase research society of Japan (ed) Handbook of amylases and related enzymes. Their sources, isolation methods, properties and applications. Pergamon Press, Oxford, p 232
Mega T, Matsushima Y (1979) Comparative studies of three exo-β-glycosidases form Aspergillus oryzae. J Biochem (Tokyo) 85:335–341
Mitsuoka T (1989) In: Mitsuoka T (ed) Methodology for research in intestinal flora. Japan Scientific Press, Tokyo, p 24
Mozaffar Z, Nakanishi K, Matsuno R, Kamikubo T (1984) Purification and properties of β-galactosidases from Bacillus circutans. Agric Biol Chem 48:3053–3061
Ogushi S, Yoshimoto T, Turn D (1980) Purification and comparison of two types of β-galactosidases from Aspergillus oryzae. J Ferment Technol 58:115–122
Perlmann GE, Longsworth LG (1948) The specific refractive increment of some purified proteins. J Am Chem Soc 70:2719–2724
Prenosil JE, Stuker E, Bourne JR (1987) Formation of oligosaccharides during enzymatic lactose. Part I: State of art. Biotechnol Bioeng 30:1019–1025
Pütter J, Becker R (1983) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3, 3rd edn. Verlag Chemie, Weinheim, p 286
Schomburg D, Saltzmann M (ed) (1991) Enzyme handbook, vol 4, 3.2.1.23. Springer, Berlin Heidelberg New York
Tanaka R, Takayama H, Morotomi M, Kuroshima T, Ueyama S, Matsumoto K, Kuroda A, Mutai M (1983) Effects of administration of TOS and Bifidobacterium breve 4006 on the human fecal flora. Bifidobact Microflora 2:17–24
Wallenfels K, Weil R (1972) β-Galactosidase. In: Boyer PD (ed) The enzymes, vol 7. Academic Press, New York, p 617
Author information
Authors and Affiliations
Additional information
Correspondence to: T. Nakayama
Rights and permissions
About this article
Cite this article
Nakao, M., Harada, M., Kodama, Y. et al. Purification and characterization of a thermostable β-galactosidase with high transgalactosylation activity from Saccharopolyspora rectivirgula . Appl Microbiol Biotechnol 40, 657–663 (1994). https://doi.org/10.1007/BF00173325
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173325