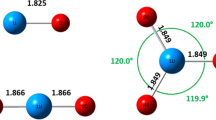
Abstract
Synthetic and natural uranium oxides UO x (2≦×≦3) have been studied with X-ray photoelectron spectroscopy (XPS) to determine the phase composition and content of uranium ions in uraninites with a varying degree of oxidation. A strong hybridization of U6p and O2s orbitals has been found which permits a quantitative assessment of the U-O bond lengths. The values of such bonds in some substances have been found to be smaller than those in synthetic U(VI) oxide. The oxides U2O5 and U3O8 contain two types of uranium ions with a varying degree of oxidation.
Similar content being viewed by others
References
Arrott A, Goldman JE (1957) Magnetic analysis of the uranium-oxygen system. Phys Rev 108:948–953
Blinova NI, Solntsev VM, Tolmachev YuM (1961) Some features of the interaction of uranium dioxide-trioxide with acids (in Russian). Dokl AN SSSR 140:1341–1316
Blinova NI, Kozhina II, Rodionova LP, Solntsev VM (1973) Solution of some uranium oxides in sulphuric acid (in Russian). Radiokhimiya 15 (No. 3):391–397
Dymkov YuM (1973) The nature of pitchblendes (in Russian) Atomizdat Moscow
Ellis DE, Rosen A, Walch PF (1975) Applications of the Dirac-Slater model to molecules. Int J Quantum Chem Symp 9:351–358
Evans S (1977) Determination of the valence electronic configuration of uranium dioxide by photoelectron spectroscopy. J Chem Soc Faraday Trans II 73:1341–1344
Frazer BC, Shirane G, Cox DE, Olsen CE (1965) Neutron-diffraction study of antiferromagnetism in UO2. Phys Rev A: 140:1448–1452
Fuggle JC, Burr AF, Watson LM, Fabian DJ, Long W (1974) X-ray photoelectron studies of thorium and uranium. J Phys F: 4:335–342
Gubanov VA, Rosen A, Ellis DE (1977) Electronic structure and bonding in ThO2 and UO2. Solid State Commun 22:219–223
Gubanov VA, Rosen A, Ellis DE (1979) Electron structure and chemical bonding in actinide oxides: monoxides and dioxides of Np, Pu, Am, Cm, and Bk. J Phys Chem Solids 40:17–28
Katz JJ, Rabinovitch E (1951) The chemistry of uranium. Part I. The element, its binary and related compound. McGraw Hill Company, Inc, New York Toronto London
Lisitsin AK (1975) The hydrogeochemistry of ore-formation (with particular reference to exogeneous epigenetic uranium ores (in Russian). Nedra Publ, Moscow
Mikhailenko VI, Kucherenko BI, Kotov MV (1973) A new method of decomposing a complex spectral contour into two symmetrical bands (in Russian) Zh Prikl Spektrosk 19:361–363
Miyake C, Sakurai H, Imoto S (1975) Photoelectron spectra of uranium (IV), (V), and (VI) complexes. Chem Phys Lett 36:158–160
Novakov T, Hollander JM (1968) Spectroscopy of inner atomic levels: electric field splitting of core p(3/2) levels in heavy atoms. Phys Rev Lett 21:1133–1136
Teterin YuA, Baranov AN, Kulakov VM, Tolmacheva NS, Nikolenko LN (1978) X-ray photoelectron studies of some nickel and cobalt compounds (in Russian) Koord Khim 4:1860–1866
Thibant E, Pireaux JJ, Riga J, Tenret-Noël C, Caudano R, Deronane EG, Verbist J (1977) Electronic structure of uranium and thorium compounds; further studies by ESCA and EPR. In: Mulak J, Suski W, Trox R (eds) Proc 2nd Int Conf on Electronic Struct Actinides 1976 Wroclaw, Poland, pp 139–144
Veal BW, Lam DJ (1974a) Bonding in uranium oxides: the role of 5f electrons. Phys Lett A. 49:466–468
Veal BW, Lam DJ (1974b) X-ray photoelectron studies of thorium, uranium, and their dioxides. Phys Rev B 10:4902–4908
Veal BW, Lam DJ, Carnall WT, Hoekstra HR (1975) X-ray photoemission spectroscopy study of hexavalent uranium compounds. Phys Rev B. 2:5651–5663
Veal BW, Lam DJ, Diamond H, Hoekstra HR (1977) X-ray photoelectron spectroscopy study of oxide of the transuranium elements Np, Pu, Am, Cm, Bk, and Cf. Phys Rev B. 15:2929–2942
Verbist J, Riga J, Pireaux JJ, Caudano R (1974) X-ray photoelectron spectra of uranium and uranium oxides, correlation with the half-life of 235Um. J Electron Spectrosc Relat Phenom 5:193–205
Walch PF, Ellis DE (1976) Effects of secondary ligand of the electronic structure of uranyls. J Chem Phys 65:2387–2392
Weber J, Gubanov VA (1979) Molecular orbital studies of the electronic structure of lanthanide and actinide complexes — III. Chemical bonding and satellite structure in the X-ray photoelectron spectrum of uranium dioxide. J Inorg Nucl Chem 41:693–699
Zhudov VI, Zelenkov AG, Kulakov VM, Odinov BV, Teterin YuA (1980) Experimental investigation of the influence of chemical surround on the conversion electrons spectrum of 235U(1/2+) — isomer (in Russian). Tezisy dokladov XXX Soveshchanija po jadernoj spektroskopii i strukture atomnogo jadra, Leningrad, Nauka, p 614
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teterin, Y.A., Kulakov, V.M., Baev, A.S. et al. A study of synthetic and natural uranium oxides by X-ray photoelectron spectroscopy. Phys Chem Minerals 7, 151–158 (1981). https://doi.org/10.1007/BF00307259
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00307259