Abstract
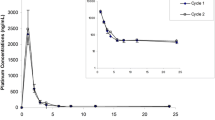
Ormaplatin is a second-generation platinum (Pt) analogue with in vitro activity against some cisplatin-resistant malignant cell lines. We have evaluated the pharmacokinetics and biotransformations of ormaplatin during a phase I trial in which ormaplatin was administered by daily 30-min infusions on 5 consecutive days every 28 days. Sixteen patients received 25 courses at doses ranging from 5.0 to 11.6 mg/m2 per day. Pharmacokinetic parameters determined for ultrafilterable Pt measured by atomic absorption spectrophotometry revealed a short half-life (t1/2 16 min), moderate volume of distribution (Vd 12 l/m2), and relatively fast systemic clearance (Cls 544 ml/min per m2). Cls and percentage of drug unbound decreased during the 5-day administration period. Average systemic exposure increased with dose; however, inter-individual variability in Cls produced overlap in systemic exposure between the dose levels. The major active biotransformation product [PtCl2(dach)] was evaluated at the highest dose level by HPLC. This product decayed monoexponentially with a mean t1/2 of 13 min and a higher degree of pharmacokinetic variability than that of ultrafilterable Pt at this dose. No uncreacted ormaplatin was detected; however, several inactive biotransformation products persisted for at least 120 min. Approximately 32% of the dose was excreted in the urine during the first day, one-third of this during the initial 1.5 h. The human pharmacokinetic characteristics of ormaplatin resemble those of cisplatin; however, additional study will be required to discern which analyte of ormaplatin correlates best with clinical effects.
Similar content being viewed by others
References
Anderson WK, Quagliato DA, Haugwitz RD, Narayanan VL, Wolpert-DeFilippes MK (1986) Synthesis, physical properties, and antitumor activity of tetraplatin and related tetra-chloroplatinum(IV) stereoisomers of 1,2-diaminocyclohexane. Cancer Treat Rep 70: 997
Andrews PA, Wung WE, Howell SB (1984) A high performance liquid chromatographic assay with improved selectivity for cisplatin and active platinum (II) complexes in plasma ultrafiltrate. Anal Biochem 143: 46
Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF (1987) Characterization of acis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res 47: 414
Borch RF, Markovitz JH, Pleasants ME (1979) A new method for HPLC analysis of Pt(II) in the urine. Anal Lett 12: 917
Campbell AB, Kalman S, Jacobs C (1983) Plasma platinum levels: relationship to cisplatin dose and nephrotoxicity. Cancer Treat Rep 67: 169
Carfagna PF, Poma A, Wyrick SD, Holbrook DJ, Chaney SG (1991) Comparisons of tetrachloro(d,l-trans) 1,2-diaminocyclo-hexaneplatinum(IV) biotransformations in the plasma of Fischer 344 rats at therapeutic and toxic doses. Cancer Chemother Pharmacol 27: 335
Chaney SG, Wyrick S, Till GK (1990) In vitro biotransformations of tetrachloro(d,l-trans) 1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in rat plasma. Cancer Res 50: 4539
Chaney SG, Gibbons GR, Wyrick SD, Podhasky P (1991) An unexpected biotransformation pathway for tetrachloro(d,l-trans)-1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in the L1210 cell line. Cancer Res 51: 969
Christian MC (1992) The current status of new platinum analogues. Semin Oncol 19: 720
Corden BJ, Fine RL, Ozols RF, Collins JM (1985) Clinical pharmacology of high-dose cisplatin. Cancer Chemother Pharmacol 14: 38
Daley-Yates PT, McBrien DCH (1984) Cisplatin metabolites in plasma, a study of their pharmacokinetics and importance in the nephrotoxic and antitumour activity of cisplatin. Biochem Pharmacol 33: 3063
Desoize B, Marechal F, Millart H, Cattan A (1991) Correlation of clinical pharmacokinetic parameters of cisplatin with efficacy and toxicity. Biomed Pharmacother 45: 203
De Waal WAJ, Maessen FJMJ, Kraak JC (1990) Analytical methodologies for the quantitation of platinum anti-cancer drugs and related compounds in biological media. J Pharmaceut Biomed Anal 8: 1
Dumas M, de Gislain C, dÁthis P, Chadoint-Noudeau V, Escousse A, Guerrin J, Autissier N (1990) Influence of hydration on ultrafilterable platinum kinetics and kidney function in patients treated withcis-diamminedichloroplatinum(II). Cancer Chemother Pharmacol 26: 278
Engineer MS, Brown NS, Ho DHW, Newman RA, Bulger RE (1989) A comparison of the effects of tetraplatin and cisplatin on renal function and getamycin pharmacology in rats. Toxicology 59: 151
Fichtinger-Schepman AMJ, van der Velde-Visser SD, van Dijk-Knijnenburg HCM, van Osterom AT, Baan RA, Berends F (1990) Kinetics of the formation and removal of cisplatin-DNA adducts in blood cells and tumor tissue of cancer patients receiving chemotherapy: comparison with in vitro adduct formation. Cancer Res 50: 7887
Forastiere AA, Belliveau JF, Goren MP, Vogel WC, Posner MR, O'Leary GP (1988) Pharmacokinetic and toxicity evaluation of five-day continuous infusion versus intermittent boluscis-diamminedichloroplatinum(II) in head and neck cancer. Cancer Res 48: 3869
Fournier C, Vennin P, Hequet B (1988) Correlation between free platinum AUC and total platinum measurement 24 h after i.v. bolus injection of cisplatin in humans. Cancer Chemother Pharmacol 21: 75
Gandara DR, DeGregorio MW, Wold H, Wilbur BJ, Kohler M, Lawrence HJ, Deisseroth AB, George CB (1986) High-dose cisplatin in hypertonic saline: reduced toxicity of a modified dose schedule and correlation with plasma pharmacokinetics. A Northern California Oncology Group pilot study in non-small-cell lung cancer. J Clin Oncol 4: 1787
Gibbons GR, Wyrick S, Chaney SG (1989) Rapid reduction of tetrachloro(d,l-trans) 1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in RPMI 1640 tissue culture medium. Cancer Res 49: 1403
Goel R, Andrews PA, Pfeifle CE, Abramson IS, Kirmani S, Howell SB (1990) Comparison of the pharmacokinetics of ultrafilterable cisplatin species detectable by derivitization with diethyldithiocarbamate or atomic absorption spectrometry. Eur J Cancer 26: 21
Harrison SD Jr, Trader MW, Dykes DJ, Plowman J, Griswold DP (1987) Schedule- and rate-dependence of tetraplatin (TP, NSC 363812) antitumor activity in L1210-bearing mice. Proc Am Assoc Cancer Res 28: 451
Hills CA, Kelland LR, Abel G, Siracky J, Wilson AP, Harrap KR (1989) Biological properties of ten human ovarian cell lines: calibration in vitro against four platinum complexes. Br J Cancer 59: 527
Ho BT, Feiffer R, Tansey LW, Newman RA, Fields WS, Krakoff IH (1987) Inhibition of brain choline acetyltransferase by tetraplatin. Brain Res Bull 19: 283
Kelland LR, Mistry P, Abel G, Loh SY, O'Neill CF, Murrer BA, Harrap KR (1992) Mechanism-related circumvention of acquiredcis-diamminedichloroplatinum(II) resistance using 2 pairs of human ovarian carcinoma cell lines by ammine/amine platinum(IV) dicarboxylates. Cancer Res 52: 3857
Kelsen DP, Alcock N, Young CW (1985) Cisplatin nephrotoxicity: correlation with plasma platinum concentrations. Am J Clin Oncol 8: 77
Lim MC, Martin RB (1976) The nature of cis amine Pd(II) and antitumorcis amine Pt(II) complexes in aqueous solutions. J Inorg Nucl Chem 38: 1911
Long DF, Repta AJ (1981) Cisplatin: chemistry, distribution and biotransformation. Biopharm Drug Dispos 2: 1
Mauldin SK, Richard FA, Plescia M, Wyrick SD, Sancar A, Chaney SG (1986) High-performance liquid chromatographic separation of platinum complexes containing thecis-1,2-diaminocyclohexane carrier ligand. Anal Biochem 157: 129
Mauldin SK, Husain I, Sancar A, Chaney SG (1986) Effects of the bidentate malonate ligand on the utilization and cytotoxicity of platinum compounds in the L1210 cell line. Cancer Res 46: 2876
Mauldin SK, Gibbons G, Wyrick SD, Chaney SG (1988) Intracellular biotransformations of platinum compounds with the 1, 2-diaminocyclohexane carrier ligand in the L1210 cell line. Cancer Res 48: 5136
Mauldin SK, Plescia M, Richard FA, Wyrick SD, Voyksner RD, Chaney SG (1988) Displacement of the bidentate malonate ligand from (d,l-trans-1,2-diaminocyclohexane)malonatoplatinum(II) by physiologically important compounds in vitro. Biochem Pharmacol 37: 3321
O'Dwyer PJ, Hudes GR, Walczak J, Schilder R, LaCreta F, Rogers B, Cohen I, Kowal C, Whitfield L, Boyd RA (1992) Phase I and pharmacokinetic study of the novel platinum analogue CI-973 on a 5-daily dose schedule. Cancer Res 52: 6746
O'Rourke T, Rodriguez G, Eckardt J, Kuhn J, Burris H, New P, Hardy J, Weiss G, Von Hoff D (1993) Neurotoxicity of ormaplatin (NSC 363812) in a phase I trial of a daily times five schedule. Proc Am Soc Clin Oncol 12: 137
Perez RP, O'Dwyer PJ, Handel LM, Ozols RF, Hamilton TC (1991) Comparative cytotoxicity of CI-973, cisplatin, carboplatin and tetraplatin in human ovarian carcinoma cell lines. Int J Cancer 48: 265
Perez RP, Perez KM, Handel LM, Hamilton TC (1992) In vitro interactions between platinum analogues in human ovarian-carcinoma cell lines. Cancer Chemother Pharmacol 29: 430
Rahman A, Roh JK, Wolpert-DeFilippes MK, Goldin A, Venditti JM, Wooley PV (1988) Therapeutic and pharmacological studies of tetrachloro(d,l-trans)-1,2-diaminocyclohexane platinum (IV) (tetraplatin), a new platinum analogue. Cancer Res 48: 1745
Reed E, Ozols RF, Tarone R, Yuspa SH, Poirier MC (1987) Platinum-DNA adducts in leukocyte DNA correlate with disease response in ovarian cancer patients receiving platinum-based chemotherapy. Proc Natl Acad Sci USA 84: 5024
Reed E, Ostchega Y, Steinberg SM, Yuspa SH, Young RC, Ozols RF, Poirier MC (1990) Evaluation of platinum-DNA adduct levels relative to known prognostic variables in a cohort of ovarian cancer patients. Cancer Res 50: 2256
Reese PA, Stafford I, Russell J, Khan M, Gill PG (1987) Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J Clin Oncol 5: 304
Riley CM, Sternson LA, Repta AJ, Seigler RW (1982) High-performance liquid chromatography of platinum complexes on solvent-generated anion exchangers III. Application to the analysis of cisplatin in urine using automated column switching. J Chromatogr 229: 373
Schmidt W, Chaney SG (1993) Role of carrier ligand in platinum resistance of human carcinoma cell lines. Cancer Res 53: 799
Smith JH, Smith MA, Litterst CL, Copley MP, Uozumi, J, Boyd MR (1988) Comparative toxicity and renal distribution of the platinum analogues tetraplatin, CHIP, and cisplatin at eqimolar doses in the Fischer 344 rat. Fund Appl Toxicol 10: 45
Smith MA, Smith JH, Litterst CL, Copley MP, Uozumi J, Boyd MR (1988) In vivo biochemical indices of nephrotoxicity of platinum analogues tetraplatin, CHIP, and cisplatin in the Fischer 344 rat. Fund Appl Toxicol 10: 62
Speer RJ, Ridway H, Hall LM, Stewart DP (1980) Preclinical testing of some cisplatin congeners as potential antitumor agents. J Clin Hemat Oncol 10: 9
Teicher BA, Holden SA, Herman TS, Stomayor EA, Khandekar V, Rosbe KW, Brann TW, Korbut TT, Frei E III (1991) Characteristics of five human tumor cell lines and sublines resistant tocis-diamminedichloroplatinum(II). Int J Cancer 47: 252
Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Robins HI, Spriggs DR (1992) Phase I trial and pharmacokinetic study of ormaplatin. Proc Am Assoc Cancer Res 33: 536
Van der Vijgh WJF, Klein I (1986) Protein binding of five platinum compounds. Cancer Chemother Pharmacol 18: 129
Vermorken JB, van der Vijgh WJF, Klein I, Hart AAM, Gall HE, Pinedo HM (1984) Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep 68: 505
Wilkoff LJ, Dulmadge EA, Trader MW, Harrison SD, Jr., Griswold DP (1987) Evaluation oftrans-tetrachloro-1,2-diaminocyclohexane platinum (IV) in murine leukemia L1210 resistant and sensitive tocis-diamminedichloroplatinum (II). Cancer Chemother Pharmacol 20: 96
Wyrick SD, Chaney SG (1987) Tritiated platinum antitumor agents containing thetrans-(d,l)-1,2-diaminocyclohexane carrier ligand. J Label Compound Radiopharm 25: 349
Author information
Authors and Affiliations
Additional information
The biotransformation studies were supported by the Upjohn Company.
Rights and permissions
About this article
Cite this article
Petros, W.P., Chaney, S.G., Smith, D.C. et al. Pharmacokinetic and biotransformation studies of ormaplatin in conjunction with a phase I clinical trial. Cancer Chemother. Pharmacol. 33, 347–354 (1994). https://doi.org/10.1007/BF00685911
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685911