Summary
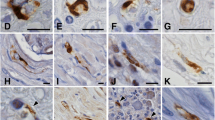
To extend earlier observations on Schwann cell remyelination in multiple sclerosis (MS) lesions (Itoyama et al. 1983) we immunostained spinal cord sections from eight Japanese MS patients with antiserum to Po glycoprotein, a major constituent of peripheral nervous system (PNS) myelin, myelin basic protein (MBP), and glial fibrillary acidic protein (GFAP). Spinal cord sections from six of the eight Japanese MS patients contained large clusters of peripheral myelin sheaths with anti-Po immunoreactivity. In lesions found in four of the six patients, thousands of Po-stained PNS myelin sheaths were present. Necrosis was prominent in these lesions which included more than half of the spinal cord's transverse area. The number and density of regenerating myelin sheaths of peripheral origin were much greater than we observed in MS spinal cord lesions of white people (Itoyama et al. 1983). Anti-GFAP immunoreactivity was present in most brain and spinal cord lesions. However, the areas in lesions that contained large groups of PNS myelin sheaths lacked anti-GFAP immunoreactivity. Our data suggest that spinal MS lesions that are large, severely demyelinated, and partially necrotic may contain factors that inhibit fibrous astrogliosis. These factors, other substances in the large lesions and/or the lack of astrocytic scarring could then promote Schwann cell invasion, multiplication, and remyelination of surviving axons.
Similar content being viewed by others
References
Adelman LS, Aronson SM (1972) Intramedullary nerve fiber and Schwann cell proliferation within the spinal cord (schwannosis). Neurology (Minneap) 22:726–731
Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43:429–435
Bignami A, Dahl D (1974) Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol 153:27–38
Blakemore WF, Patterson RC (1975) Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol 4:573–585
Blakemore WF (1976) Invasion of Schwann cells into the spinal cord of the rat following local injections of lysolecithin. Neuropathol App Neurobiol 2:21–39
Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28:351–354
Everly JL, Brady RO, Quarles RH (1973) Evidence that the major protein in rat sciatic nerve myelin is a glycoprotein. J Neurochem 21:329–344
Feigin I, Popoff N (1966) Regeneration of myelin in multiple sclerosis: the role of mesenchymal cells in such regeneration and in myelin formation in the peripheral nervous system. Neurology (Minneap) 16:364–372
Feigin I, Ogata J (1971) Schwann cells and peripheral myelin within human central nervous tissues: the mesenchymal character of Schwann cells. J Neuropathol Exp Neurol 30:603–612
Ghatak NR, Hirano A Doron Y, Zimmerman HM (1973) Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol 29:262–267
Harrison BM, McDonald WI, Ochoa J (1972) Remyelination in the central diphtheria toxin lesion. J Neurol Sci 16:293–302
Hirano A, Zimmerman HM, Levine S (1969) Electron-microscopic observations of peripheral myelin in a central nervous system lesion. Acta Neuropathol (Berl) 12:348–365
Ikuta F, Koga M, Takeda S, Ohama E, Takeshita I, Ogawa H, Wang M (1982) Comparison of MS pathology between 70 American and 75 Japanese autopsy cases. In: Kuroiwa Y, Kurland LT (eds) Multiple sclerosis, East and West. Kyushu University Press, Fukuoka, pp 297–306
Itoyama Y, Sternberger NH, Kies MW, Cohen SR, Richardson EP, Jr, Webster H deF (1980a) Immunocytochemical method to identify myelin basic protein in oligodendroglia and myelin sheaths of the human nervous system. Ann Neurol 7:157–166
Itoyama Y, Sternberger NH, Webster H deF, Quarles RH, Cohen SR, Richardson EP, Jr (1980b) Immunocytochemical observation on the distribution of myelin-associated glycoprotein and myelin basic protein in multiple sclerosis lesions. Ann Neurol 7:167–177
Itovama Y, Webster H deF, Richardson EP, Jr, Trapp BD (1983) Schwann cell remyelination of demyelinated axons in spinal cord multiple sclerosis. Ann Neurol 14:339–346
Itoyama Y, Ohnishi A, Tateishi J Kuroiwa Y, Webster H deF (1984) Immunocytochemical study of Schwann cell remyelination in spinal cord lesions of Japanese multiple sclerosis patients. In: Sobue I (ed) Peripheral neuropathy. Proceedings of the International Symposium on Peripheral Neuropathy, Excerpta Medica, Tokyo (in press)
Kuroiwa Y, Igata A, Itahara K, Koshijima S, Tsubaki T, Toyokura Y, Shibasaki H (1975) Nationwide survey of multiple sclerosis in Japan. Clinical analysis of 1,084 cases. Neurology (Minneap) 25:845–851
Lassmann H, Kitz K, Wisniewski HM (1980) Structural variability of demyelinating lesions in different models of subacute and chronic experimental allergic encephalomyelitis. Acta Neuropathol (Berl) 51:191–201
Newcombe J, Cuzner ML, Roytta M, Frey H (1980) White matter proteins in multiple sclerosis. J Neurochem 34:700–708
Ogata J, Feigin I (1975) Schwann cell and regenerated peripheral myelin in multiple sclerosis: an ultrastructural study. Neurology (Minneap) 25:713–716
Prineas JW, Connell F (1979) Remyelination in multiple sclerosis. Ann Neurol 5:22–31
Raine CS (1976) On the occurrence of Schwann cells within the normal central nervous system. J Neurocytol 5:371–380
Raine CS, Traugott U, Stone SH (1978) Glial bridges and Schwann cells migration during chronic demyelination in the CNS. J Neurocytol 7:541–553
Reier PJ, Webster H deF (1974) Regeneration and remyelination of Xenopus tadpole optic nerve fibers following transection or erush. J Neurocytol 3:591–618
Schober R, Itoyama Y, Sternberger NH, Trapp BD, Richardson EP, Jr, Asbury AK, Quarles RH, Webster H deF (1981) Immunocytochemical study of Po glycoprotein, P1 and P2 basic protein, and myelin-associated glycoprotein (MAG) in lesions of idiopathic polyneuritis. Neuropathol Appl Neurobiol 7:421–434
Shibasaki H, McDonald WI, Kuroiwa Y (1981) Racial modification of clinical picture of multiple sclerosis: Comparison between British and Japanese patients. J Neurol Sci 49:253–271
Shiraki H (1968) The comparative study of rabies postvaccinal encephalomyelitis and demyelinating encephalomyelitides of unknown origin, with special reference to the Japanese cases. In: Bailey OT, Smith DE (eds) The central nervous system some experimental models of neurological diseases. Williams and Wilkins, Baltimore, pp 87–123
Snyder, DH, Valsamis MP, Stone SH, Raine CS (1975) Progressive and reparatory events in chronic experimental allegic encephalomyelitis. J Neuropathol Exp Neurol 34:209–221
Sternberger LA, Hardy Ph, Jr, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunocytochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Sternberger LA, Hardy PH, Jr, Cuculis JJ, Meyer HG (1970) The The unlabeled antibody enzyme method of immunomyelin-forming oligodendrocytes of newborn rat CNS. J Neurocytol 7:251–263
Sternberger NH, Itoyama Y, Kies MW, Webster H deF (1978b) Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci USA 75:2521–2524
Trapp BD, McIntyre LJ, Quarles RH, Sternberger NH, Webster H deF (1979) Immunocytochemical localization of rat peripheral nervous system myelin proteins: P2 protein is not a component of all peripheral nervous system myelin sheaths. Proc Natl Acad Sci USA 76:3552–3556
Trapp BD, Itoyama Y, Sternberger NH, Quarles RH, Webster H deF (1981) Immunocytochemical localization of P0 protein in Golgi complex membranes and myelin of developing rat Schwann cells. J Cell Biol 90:1–6
Webster H deF, Trapp BD, Sternberger NH (1981) Myelinforming glial cells: morphological and immunocytochemical observations. In: Garrod DR (ed) Development in the nervous system. Cambridge University Press, Cambridge, pp 265–288
Wood JG, Dawson RMC (1973) A major myelin glycoprotein of sciatic nerve. J Neurochem 21:717–719
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Itoyama, Y., Ohnishi, A., Tateishi, J. et al. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein (GFAP). Acta Neuropathol 65, 217–223 (1985). https://doi.org/10.1007/BF00687001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687001