Summary
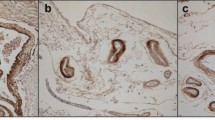
The structure of partially purified, CNS amyloid fibrils from three different sources have been compared by negative stain EM. The fibrils isolated from brains with senile dementia of Alzheimer type were 4–8 nm in diameter, narrowing every 30–40 nm and apparently composed of two 2–4 nm filaments. The fibrils from a Gerstmann-Sträussler syndrome brain were 7–9 nm in diameter, narrowing every 70–80 nm and with a suggestion that they are composed of two 3–5 nm filaments. The fibrils isolated from 87V scrapie-affected mouse brains were 4–8 nm in diameter with a twist every 15–25 nm presumably composed of two 2–4 nm filaments. The fibrils from the scrapie brains were usually observed in pairs. The shape of the clusters of the isolated amyloid fibrils observed in each disease was similar in negative stain and thin section EM preparations and was related to the characteristic morphology of the amyloid fibrils in the neuritic and amyloid plaques in situ. The structural differences between the CNS amyloid fibrils from the various diseases studied by us may reflect differences in the polypeptides which comprise the fibril and/or a different pathogenesis in the formation of the amyloid fibrils.
Similar content being viewed by others
References
Beck E, Daniel PM, (1979) Kuru and Creutzfeldt-Jakob disease: neuropathological lesions and their significance. In: Prusiner SB, Hadlow WJ (eds) Slow transmissible diseases of the nervous system, vol 1. Academic Press, New York, pp 253–285
Brown P, Salazar AM, Gibbs CJ Jr, Gajdusek DD (1982) Alzheimer's disease and transmissible virus dementia (Creutzfeldt-Jakob disease). Ann NY Acad Sci 396:131–143
Bruce ME, Fraser H (1975) Amyloid plaques in the brains of mice infected with scrapie, morphological variation and staining properties. Neuropathol Appl Neurobiol 1:189–202
Bruce ME, Fraser H (1981) Effect of route of infection on the frequency and distribution of cerebral amyloid plaques in scrapie mice. Neuropathol Appl Neurobiol 7:289–298
Chandler RL (1963) Experimental scrapie in the mouse. Res Vet Sci 4:276–285
Eikelenboom P, Stam FC (1982) Immunoglobins and complement factors in senile plaques. Acta Neuropathol (Berl) 57:235–242
Fraser H (1979) Neuropathology of scrapie: the precision of the lesions and their diversity. In: Prusiner SB, Hadlow WJ (eds) Slow transmissible diseases of the nervous system, vol 1. Academic Press, New York, pp 387–406
Gajdusek DC (1977) Unconventional viruses and the origin and disappearance of kuru. Science 197:943–960
Glenner GG, Eanes ED, Bladen HA, Finke RP, Termine JD (1974) B pleated sheet fibrils: a comparison of native amyloid fibrils with synthetic protein fibrils. J Histochem Cytochem 22: 1141–1158
Glenner GG, Page DL (1976) Amyloid, amyloidosis and amyloidogenesis. In: Rechter GW, Epstein MA (eds) International review of experimental pathology, vol 15. Academic Press, New York, pp 1–92
Goudsmit J, Morrow CH, Asher DM, Yanagikara RT, Masters CL, Gibbs CJ Jr, Gajdusek DC (1980) Evidence for and against the transmissibility of Alzheimer's disease. Neurology 30:945–950
Jervis GA, Soltz SW (1936) Alzheimer's disease—the so-called juvenile type. Am J Psychiat 93:39–56
Klatzo V, Gajdusek DC, Zigas V (1959) Pathology of kuru. Lab Invest 8:799–847
Masters CL, Gajdusek DC, Gibbs CJ Jr (1981) Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome: With an analysis of the various forms of amyloid plaque deposition in the virus induced spongiform encephalopathies. Brain 104:559–588
Merz PA, Somerville RA, Wisniewski HM, Iqbal K (1981) Abnormal fibrils from scrapie-infected brain. Acta Neuropathol (Berl) 54:63–74
Narang HK (1980) High resolution election microscopic analysis of the amyloid fibril in Alzheimer's disease. J Neuropathol Exp Neurol 39:621–631
Powers JM, Schlaepfer WW, Wellingham MC, Hall BJ (1981) An immunoperoxidase study of senile cerebral amyloidosis with pathogenetic consideration. J Neuropathol Exp Neurol 40:592–612
Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin EC (1968) The characterization of soluble amyloid prepared in water. J Clin Invest 47: 924–933
Rosenthal CJ, Franklin EC (1977) Amyloidosis and amyloid protein. In: Thompson R (ed) Recent advances in clinical immunology. Churchill-Livingstone, Edinburgh, pp 41–76
Schlote W, Boellaard JW, Schumm F, Stohr M (1980) Gerstmann-Sträussler Scheinkers disease: electron-microscopic observations on a brain biopsy. Acta Neuropathol (Berl) 52:203–211
Shirahama T, Cohen AS (1965) Structure of amyloid fibrils after negative staining and high resolution electron microscopy. Nature (Lond) 206:737–738
Shirahama T, Skinner M, Westermark P, Rubinow A, Cohen AS, Brun A, Kemper TL (1982) Senile cerebral amyloid prealbumin as a common constituent in the neuritic plaque, in the neurofibrillary tangle, and in the microangiopathic lesion. Am J Pathol 107:41–50
Sorenson GD, Finke E (1968) The ultrastructure of amyloid. In: Mandema E, Ruinen L, Scholten JH, Cohen AS (eds) Amyloidosis. Excerpta Medica, Amsterdam, pp 184–190
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Terry RD, Wisniewski HM (1970) The ultrastructure of the neurofibrillary tangle and the senile plaque. In: Wolstenholmer GEW, O'Connor M (eds) CIBA Foundation Symposium on Alzheimer's disease and related conditions, Churchill, London, pp 145–168
Vaughan DW, Peters A (1981) The structure of neuritic plaques in the cerebral cortex of aged rats. J Neuropathol Exp Neurol 40:472–487
Vorbrodt AW, Lossinsky AS, Wisniewski HM, Moretz RC, Iwanowski L (1981) Ultrastructural cytochemical studies of cerebral microvasculature in scrapie infected mice. Acta Neuropathol (Berl) 53:203–211
Wisniewski HM, Johnson AB, Raine CS, Kay WJ, Terry RD (1970) Senile plaques and cerebral amyloidosis in aged dogs. Lab Invest 23:287–296
Wisniewski HM, Ghetti B, Terry RD (1973) Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol 32:566–584
Wisniewski HM, Terry RD (1973) Re-examination of the pathogenesis of the senile plaque. In: Zimmerman HM (ed) Progress in neuropathology, vol 11. Grune and Stratton, New York, pp 1–26
Wisniewski HM, Bruce ME, Fraser H (1975) Infection etiology of neuritic (senile) plaques in mice. Science 190: 1108–1110
Wisniewski HM, Terry RD (1976) Neuropathology of the aging brain. In: Terry RD, Gershon S (eds) Neurobiology of aging. Raven Press, New York, pp 265–280
Wisniewski HM, Moretz RC, Lossinsky AL (1981a) Evidence of induction of localized amyloid deposits and neuritic plaques by an infectious agent. Ann Neurol 10:517–522
Wisniewski HM, Sinatra RS, Iqbal K, Grundke-Iqbal (1981b) Neurofibrillary and synaptic pathology in the aged brain. In: Johnson JE (ed) Aging and cell structure. Plenum Press, New York, pp 105–141
Wisniewski HM, Kozlowski P (1982) Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT). Ann NY Acad Sci 396:119–129
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Merz, P.A., Wisniewski, H.M., Somerville, R.A. et al. Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol 60, 113–124 (1983). https://doi.org/10.1007/BF00685355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685355