Summary
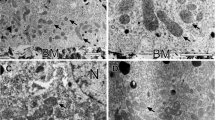
The macular mottled mouse is a murine model of the kinky hair syndrome, characterized by a deficiency in copper transport. Cytochromec oxidase (CCO), a respiratory enzyme, is located in the inner mitochondrial membrane and consists of seven subunits, along with copper and iron. Biochemical and histochemical findings indicated that CCO activity was decreased in the cerebellum of the macular mottled mice but not in that of the controls. Immunocytochemical analysis, using anti-CCO and anti-complex III rabbit sera, revealed that CCO in the macular mottled mice was stained more weakly than that in the controls. Immuno-electron microscopic examination of CCO and complex III, using a method of gold labeling, was also performed. In the control mice, a high concentration of gold particles present over CCO and complex III could be seen in the inner mitochondrial membrane. The number of CCO-labeled gold particles was remarkably less, however, in the macular mottled mice, while no significant difference was found in the labeling of complex III between the two groups. It may concluded that the very low CCO enzyme content in the macular mottled mouse results not only from a copper transport disorder but also from a CCO protein synthesis disorder which impairs the localization of CCO protein in the cerebellum.
Similar content being viewed by others
References
Aquilar MJ, Chadwick DL, Okuyama K, Kamoshita S (1966) Kinky hair disease. 1. clinical and pathological features. J Neuropathol Exp Neurol 25:507–522
Camakaris J, Phillips M, Danks DM, Brown R, Stevenson T (1983) Mutations in humans and animals which affect copper metabolism. J Inherited Metab Dis [Suppl 1] 6:44–50
French JH, Sherard ES, Lubell H, Brotz M, Moore CL (1972) Trichopoliodystrophy. Report of a case and biochemical studies. Arch Neurol 26:229–244
Ghatak NR, Hirano A, Poon TP, French JH (1972) Trichopoliodystrophy. II. Pathologic changes in skeletal muscle and nervous system. Arch Neurol 26:60–72
Hatefi Y, Rieske JS (1967) The preparation and properties of DPNH-cytochrome c reductase (complex I–III of the respiratory chain). Methods Enzymol 10:225–231
Holtzman NA (1976) Menkes kinky hair syndrome. A genetic disease involving copper. Fed Proc 35:2276–2280
Horn N (1984) Copper metabolism in Menkes disease. In: Rennert OM, Chan WY (eds) Genetic implications, vol 2. Boca Raton, Florida, pp 25–52
Hunt DM (1974) Primary defect in copper transport underlies mottled mutants in the mouse. Nature 249:852–854
King TE (1967) Preparation of succinate-cytochrome c reductase. Methods Enzymol 10:216–225
Liu HC, Chan WY, Rennert OM (1982) Histochemical studies of fibroblasts from patients with Menkes kinky hair disease and Wilsons disease. Histochem J 14:781–789
Menkes JH, Alter M, Steigleder GK, Weakly DR, Sung JH (1962) A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics 29:764–779
Nagara H, Yajima K, Suzuki K (1980) An ultrastructural study on the cerebellum of the brindled mouse. Acta Neuropathol (Berl) 52:41–50
Prohaska JR, Smith TL (1982) Effect of dietary or genetic copper deficiency on brain catecholamines, trace metals and enzymes in mice and rats. J Nutr 112:1706–1717
Rezek DL, Moore CL (1977) Depletion of cytochrome oxidase in brain mitochondria from the mottled mouse mutant. Soc Neurosci Abstr 3:51
Rezek DL, Moore CL (1986) Depletion of brain mitochondria cytochrome oxidase in the mottled mouse mutant. Exp Neurol 91:640–645
Roth J, Bendayan M, Orci L (1978) Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem 26:1074–1081
Sato, T, Anno M, Arahata K, Nakamura S, Mukoyama M, Nakamura H, Ozawa T (1986) Immuno-cytochemical demonstration of cytochrome c oxidase and complex III in tissues from patients with mitochondrial encephalomyopathy. Muscle Nerve [Suppl] 9:183
Sato T, Anno M, Nakamura S, Uchida T, Ozawa T (1987) Colloidal-gold labeling of electron transport enzymes in mitochondrial myopathy for immuno-electron microscopy. Shinkei Kenkyu No Shinpo 31:646–652
Sekuzu I, Orri Y, Ohnishi K (1965) Purification and assay of cytochromes. Protein, nucleic acid enzyme (Tokyo) 10:1610–1620
Seligman AM, Karnousky MJ, Wasserkrug HL, Hanker JS (1968) Non-droplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol 38:1–14
Wielburski A, Nelson BD (1983) Evidence for the sequential assembly of cytochrome oxidase subunits in rat liver mitochondria. Biochem J 212:829–834
Wielburski A, Nelson BD (1984) Heme a induces assembly of rat liver cytochrome c oxidase subunits I–III in isolated mitochondria. FEBS lett 177:291–294
Yajima K, Suzuki K (1979) Neuronal degeneration in the brain of the brindled mouse. An ultrastructural study of the cerebral cortical neurons. Acta Neuropathol (Berl) 45:17–25
Yajima K, Suzuki K (1979) Neuronal degeneration in the brain of the brindled mouse. A light microscope study. J Neuropathol Exp Neurol 38:35–46
Yamano T, Shimada M, Kawasaki H, Onaga A, Nishimura M (1987) Clinico-pathological study on macular mutant mouse. Acta Neuropathol (Berl) 72:256–260
Yoshimura N (1988) Neuronal degeneration in the brain of the brindled mouse. Histochemical demonstration of decreased cytochrome oxidase activity in the cerebellum and brain stem. Acta Pathol Jpn 38:705–712
Yoshimura N, Kudo H (1983) Mitochondrial abnormalities in Menkes kinky hair disease (MKHD) electron microscopic study of the brain from an autopsy case. Acta Neuropathol (Berl) 59:295–303
Author information
Authors and Affiliations
Additional information
Supported by grants from the Ministry of Education and Ministry of Health and Welfare of Japan and by a special Japanese government Grant-in-Aid
Rights and permissions
About this article
Cite this article
Seki, K., Sato, T., Ishigaki, Y. et al. Decreased activity of cytochromec oxidase in the macular mottled mouse: an immuno-electron microscopic study. Acta Neuropathol 77, 465–471 (1989). https://doi.org/10.1007/BF00687247
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687247