Summary
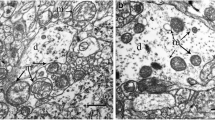
The acute cortical response to surgical brain isolation and subsequent extracorporal normoxic or 30 min hypoxic (PaO2=20 mm Hg) perfusions (hypoxic hypoxia with relative ischemia) was evaluated. Cerebral blood flow, arterial pH and CO2 were maintained constant during both perfusions; only the arterial oxygen content was changed. The isolated brain model used in this and previous investigations produces no qualitative ultrastructural changes in the neocortex following brain isolation and normoxic perfusion. However, the acute cortical structural response to 30 min of hypoxic hypoxia with relative ischemia demonstrated a number of important observations. Hypoxic hypoxia produced ultrastructural responses common to cerebral ischemia such as nuclear chromatin clumping, nucleolar condensation and cytoskeletal breakdown. Although neuronal abnormalities seen after 30 min of hypoxic hypoxia were similar to those acute neuronal changes observed following complete cerebral ischemia without recirculation, they differed three ways: (a) mitochondrial swelling and microvacuolation were observed in many cortical pyramidal neurons. (b) Glycogen particles within astroglial processes were observed even after a 30-min period of hypoxic hypoxia. (c) Perivascular astroglial swelling was minimal despite considerable perineuronal swelling. In contrast, incomplete cerebral ischemia produces mitochondrial changes similar to those in hypoxic hypoxia but also causes the depletion of tissue glycogen and perivascular glial swelling. Thus, hypoxic hypoxia with relative ischemia produces a unique acute ultrastructural response compared to either complete or incomplete cerebral ischemia.
Similar content being viewed by others
References
Aaronson RP, Woo E (1981) Organization in the cell nucleus: divalent cations modulate the distribution of condensed and diffuse chromatin. J Cell Biol 90:181–186
Agardh C-D, Kalimo H, Olsson Y, Siesjö BK (1980) Hypoglycemic brain injury. I. Metabolic and light microscopic findings in rat cerebral cortex during profound insulin-induced hypoglycemia and in the recovery period following glucose administration. Acta Neuropathol (Berl) 50:31–41
Agardh C-D, Kalimo H, Olsson Y, Siesjö BK (1981) Hypoglycemic brain injury: metabolic and structural findings in rat cerebellar cortex during profound insulin-induced hypoglycemia and in the recovery period following glucose administration. J Cereb Blood Flow Metab 1:71–84
Bouteille M, Laval M, Dupuy-Coin AM (1974) Localization of nuclear functions as revealed by ultrastructural autoradiography and cytochemistry. In: Busch H (ed) The cell nucleus, vol 1. Academic Press, New York London, pp 5–64
Brierley JB, Medrum BS, Brown AW (1973) The threshold and neuropathology of cerebral “anoxic-ischemic” cell changes. Arch Neurol 29:267–374
Brown AW, Brierley JB (1972) Anoxic-ischemic cell changes in rat brain. Light microscopic and fine-structural observations. J Neurol Sci 16:59–84
Brown AW, Brierley JB (1973) The earliest alterations in rat neurones and astrocytes after anoxia-ischemia. Acta Neuropathol (Berl) 23:9–22
Bryan RM, Jobsis FR (1986) Insufficient supply of reducing equivalents to the respiratory chain in cerebral cortex during severe insulin-induced hypoglycemia in cats. J Cereb Blood Flow Metab 6:286–291
Drewes LR, Gilboe DD, Betz AL (1973) Metabolic alterations in brain during anoxic-anoxia and subsequent recovery. Arch Neurol 29:385–390
Fitzpatrick JH, Gilboe DD (1982) Effects of nitrous oxide on the cerebrovasculature tone, oxygen metabolism and electroencephalogram of the isolated perfused canine brain. Anesthesiology 57:480–484
Fitzpatrick JH, Gilboe DD, Drewes LR, Betz AL (1976) Relationship of cerebral oxygen uptake to EEG frequency in isolated canine brain. Am J Physiol 231:1840–1846
Gilboe DD, Betz AL, Langebartel DA (1973) A guide for the isolation of the canine brain. J Appl Physiol 34:534–537
Gilboe DD, Kintner DB, Emoto SE, Fitzpatrick JH (1986) Intracellular pH and hypoxic damage in the canine brain. In: Krieglstein J (ed) Pharmacology of cerebral ischemia. Elsevier, Amsterdam New York Oxford, pp 119–129
Hertz L (1981) Features of astrocytic function apparently involved in the response of central nervous tissue to ischemia hypoxia. J Cereb Blood Flow Metab 1:143–153
Ignelzi RJ, Schmickroth C (1976) The unique response of brain nuclei to ischemia. In: Austin GM (ed) Contemporary aspects of cerebrovascular disease. Professional Information Library, Dallas, pp 228–232
Jenkins LW, Povlishock JT, Becker DP, Miller JD, Sullivan HG (1979) Complete cerebral ischemia: an ultrastructural study. Acta Neuropathol (Berl) 48:113–125
Jenkins LW, Povlishock JT, Lewelt W, Miller JD, Becker DP (1981) The role of post-ischemic recirculation in the development of ischemic neuronal injury following complete cerebral ischemia. Acta Neuropathol (Berl) 55:205–220
Jenkins LW, Becker DP, Coburn TH (1984) A quantitative analysis of perivascular glial swelling and ischemic neuronal injury. In: Go KG (ed) Recent progress in the study and therapy of brain edema. Plenum Press, New York, pp 123–137
Kalimo H, Paljarvi L, Vapalahti M (1979) The early ultrastructural alterations in the rabbit cerebral and cerebellar cortex after compression ischemia. Neuropathol Appl Neurobiol 5:211–223
Kalimo H, Agardh C-D, Olsson Y, Siesjö BK (1980) Hypoglycemic brain damage. II. Electron-microscopic findings in rat cerebral cortical neurons during profound insulin-induced hypoglycemia and in the recovery period following glucose administration. Acta Neuropathol (Berl) 50:43–52
Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjö BK (1981) Brain lactic acidosis and ischemic cell damage. 2. Histopathology. J Cereb Blood Flow Metab 1:313–327
Kalimo H, Olsson Y, Paljarvi L, Soderfeldt B (1982) Structural changes in brain tissue under hypoxic-ischemic conditions. J Cereb Blood Flow Metab 2 [Suppl 1]:19–22
Kintner D, Fitzpatrick JH, Louie JA, Gilboe DD (1984) Cerebral oxygen and energy metabolism during and after 30 min of moderate hypoxia. Am J Physiol 247:E475-E482
Lehninger AL, Fiskum G, Vercesi A, Tew W (1981) Ca2+ transport by mitochondria: a survey. In: Bronner F, Peterlik M (eds) Calcium and phosphate transport across biomembranes. Academic Press, New York, pp 73–78
Levine S (1960) Anoxic-ischemic encephalopathy in rats. Am J Pathol 36:1–17
Lewelt W, Jenkins LW, Miller JD (1982) Effects of experimental fluid percussion injury of the brain on cerebrovascular reactivity to hypoxia and to hypercapnia. J Neurosurg 56:332–338
Lynch G, Baudry M (1984) The biochemistry of memory: a new and specific hypothesis. Science 224:1057–1063
Margolis RL (1983) Calcium and microtubules. In: Cheung WY (ed) Calcium and cell function, vol 4. Academic Press, New York, pp 313–329
McGee-Russel SM, Brown AW, Brierley JB (1970) A combined light and electron microscope study of early anoxic-ischaemic cell change in rat brain. Brain Res 20:193–200
Paljarvi L, Soderfeldt B, Kalimo H, Olsson Y, Siesjö BK (1982) The brain in extreme respiratory acidosis. A light and electron microscopic study in the rat. Acta Neuropathol (Berl) 58:87–94
Paljarvi L, Rehncrona S, Soderfeldt B, Olsson Y, Kalimo H (1983) Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol (Berl) 60:232–240
Rasmussen H (1983) Pathways of amplitude and sensitivity modulation in the calcium messenger system. In: Cheung WY (ed) Calcium and cell function, vol 4. Academic Press, New York, pp 2–27
Rehncrona S, Rosen I, Siesjö BK (1981) Brain lactic acidosis and ischemic cell damage. 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab 1:297–311
Salford LG, Plum F, Brierley JB (1973) Graded hypoxia ischemia in rat brain. II. Neuropathological alterations and their implications. Arch Neurol 29:234–238
Siesjö BK (1978) Brain energy metabolism. Wiley & Sons, New York, pp 398–452
Siesjö BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1:155–185
Siesjö BK, Wieloch T (1985) Molecular mechanisms of ischemic brain damage: calcium related events. In: Plum F, Pulsinelli W (eds) Cerebrovascular diseases. Raven Press, New York, pp 187–197
Siesjö BK, Wieloch T (1985) Brain injury: neurochemical aspects. In: Becker DP, Povlishock JT (eds) Central nervous system trauma status report 1985. William Byrd Press, Richmond, pp 513–532
Simon RP, Griffiths T, Evans MC, Swan JH, Meldrum BS (1984) Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J Cereb Blood Flow Metab 4:350–361
Author information
Authors and Affiliations
Additional information
Supported by an NIH grant NS05961
Rights and permissions
About this article
Cite this article
Allen, A., Yanushka, J., Fitzpatrick, J.H. et al. Acute ultrastructural response of hypoxic hypoxia with relative ischemia in the isolated brain. Acta Neuropathol 78, 637–648 (1989). https://doi.org/10.1007/BF00691291
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00691291