Summary
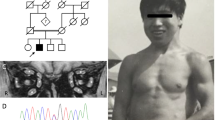
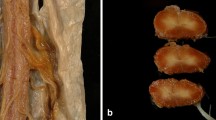
An autopsy case of hereditary peroneal muscular atrophy (PMA) with rigidity and static tremor is presented. The patient developed slowly progressive distal muscular atrophy of the legs at the age of 15 years. By the age of 52 years, PMA became marked associated with pes cavus, and tremor and rigidity of the extremities were noted. Motor and sensory conduction velocities gradually depressed and lost near the end of his life. At autopsy, the major neuropathological abnormalities involved the peripheral nervous systems, and were characterized by axonal atrophy and loss of myelinated fibres. These changes involved both the proximal and distal nerves, being more severely affected in the distal. The pathological changes in other regions of the nervous systems were mainly confined to the spinal cord, dorsal ganglia and spinal nerve roots, and pigmented neurons in the brain stem. Morphometrically, the total fascicular area was much smaller than in control, but the total number of myelinated fibers greatly outnumbered that of control 75 200 to 48 200 at the proximal sciatic nerve and then gradually decreased towards the periphery; however, even in the distal sural nerve, the total number of myelinated fibers exceeded that of control (6820 to 5469). Thus, the density of myelinated fibers were much higher, being 1.5 to 2 times greater, than in control. Its abrupt decline at the distal nerve might account for neurogenic atrophy of the distal musculature. Unmyelinated fibers were slightly increased in density and not atrophic. This case is unique in its clinicopathology and does not belong to any subtypes of PMA including “neuronal plus”.
Similar content being viewed by others
References
Behse F, Buchthal F (1977) Peroneal muscular atrophy (PMA) and related disorders. II. Histological findings in sural nerves. Brain 100:67–85
Bradley WG, Madrid R, Davis CJF (1977) The peroneal muscular atrophy syndrome. Clinical, genetic, electrophysiological and nerve biopsy studies. Part 3. Clinical, electrophysiological and pathological correlations. J Neurol Sci 32:123–136
Buchthal F, Behse F (1977) Peroneal muscular atrophy (PMA) and related disorders. I. Clinical manifestations as related to biopsy findings, nerve conduction and electromyography. Brain 100:41–66
Dupuis M, Brucher JM, Gonsette R (1983) Clinico-anatomical report of a case of neuronal type of Charot-Marie-Tooth disease. Rev Neurol (Paris) 139:643–649
Dyck PJ (1975) Inherited neuronal degeneration and atrophy affecting peripheral motor, sensory, and autonomic neurons. In: Dyck PJ, Thomas PK, Lambert EH (eds) Peripheral neuropathy Saunders Co., London, pp 825–867
Dyck PJ, Lambert EH (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. 1. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch Neurol 18:603–618
Dyck PJ, Lambert EH (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. 2. Neurologic, genetic, and electrophysiologic findings in various neuronal degenerations. Arch Neurol 18:619–625
Gillespie MJ, Stein RB (1983) The relationship between axon diameter, myelin thickness and conduction velocity during atrophy mammalian peripheral nerves. Brain Res 259:41–56
Hashimoto K, Kawai M, Sakuta M (1988) A case of sporadic polyneuropathy with tomacula demonstrating marked nerve conduction delay. Clin Neurol (Tokyo) 28:1023–1029
Hughes JT, Brownell B (1972) Pathology of peroneal muscular atrophy (Charcot-Marie-Tooth disease). J Neurol Neurosurg Psychiatry 35:648–657
Itoh Y, Yagishita S, Ono S, Nakano T (1978) Ultrastructural study on postmortem changes of the peripheral nerve. J Clin Electron Microsc 11:347–360
Julien Y, Vital C, Vallat JM, Coquet M, Le Blance M (1974) Maladie de Charcot-Marie-Tooth et diabéte. Étude clinique, ultrastructural et autopsique d'une observation. Nouv Presse Med 3:139–142
Low PA, McLeod JG, Prineas JW (1978) Hypertrophic Charcot-Marie-Tooth disease. Light and electron microscope studies of the sural nerve. J Neurol Sci 35:93–115
Madrid R, Bradley WG (1975) The pathology of neuropathies with focal thickning of the myelin sheath (Tomaculous neuropathy). Studies on the formation of the abnormal myelin sheath J Neurol Sci 25:415–448
Madrid R, Bradley WG, Davis CJF (1977) The peroneal muscular atrophy syndrome. Clinical, genetic, electrophysiological and nerve biopsy studies. Part 2. Observations on pathological changes in sural nerve biopsies. J Neurol Sci 32:91–122
Mathews T, Moossy J (1972) Mixed glioma, multiple sclerosis, and Charcot-Marie-Tooth disease. Arch Neurol 27:263–268
Oguchi K, Tsubaki T, Ikuta F (1977) An autopsy case of Charcot-Marie-Tooth disease associated with optic nerve atrophy, posterior columns and spinocerebellar degeneration. Consideration on a family of herediatary muscular atrophy with ataxia, retinitis pigmentosa and diabetes mellitus. Clin Neurol (Tokyo) 17:52–57
O'Neill JH, Gilliatt RW (1987) Adaptation of the myelin sheath during axonal atrophy. Acta Neuropathol (Berl) 74:62–66
Smith TW, Bhawan J, Keller BB, DeGirolami U (1980) Charcot-Marie-Tooth disease associated with hypertrophic neuropathy. A neuropathologic study of two cases. J Neuropath Exp Neurol 39:420–440
Thomas PK, Calne BD (1974) Motor nerve conduction velocity in peroneal muscular atrophy: evidence for genetic heterogeneity. J Neurol Neurosurg Psychiatry 37:68–75
Tredici G, Petruccuili-Pizzini MG, Gergely A, Coletti A (1984) The importance of quantitative electron microscopy in studying hypertrophic neuropathies. A comparison between a case of Déjérine-Sottas disease (HMSN III) and a case of the hypertrophic form of Charcot-Marie-Tooth disease (HMSN I). Int J Tissue React 6:267–274
Vallat JM, Gil R, Leboutet MJ, Hugon J, Moulies D (1987) Congenital hypo- and hypermyelination neuropathy. Two cases. Acta Neuropathol (Berl) 74:197–201
Van Weerden TW, Jan Houthoff H, Sie O, Minderhoud JM (1982) Variability in nerve biopsy findings in a kinship dominantly inherited Charcot-Marie-Tooth disease. Muscle Nerve 5:185–196
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Itoh, Y., Yagishita, S., Amano, N. et al. An autopsy case of peroneal muscular atrophy with rigidity and tremor. Acta Neuropathol 80, 671–679 (1990). https://doi.org/10.1007/BF00307638
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307638