Summary
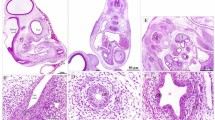
The aim of this investigation was to determine whether volumetric changes occurred in differentiating layers of stratified squamous epithelium. Samples of cheek pouch mucosa from 5 hamsters were obtained, prepared for electron microscopy using carefully controlled methods, and electron micrographs obtained from defined basal, spinous and granular layers of the epithelium. Stereological point counting procedures were used to determine the ratio of nucleus to cytoplasm for each of the defined cell layers. From direct measurement of nuclear profiles, major and minor axes were transformed to diameters of circles of equivalent area and it was thus possible to obtain an estimate of nuclear volume. Using the previously determined nuclear-cytoplasmic ratios the volume of cytoplasm and hence cell volume, could be estimated for the cell layers. Between basal and granular layers, nuclear-cytoplasmic ratios decreased from 0.42 to 0.08, whereas cytoplasmic and cellular volumes increased progressively from 248 to 1052 μm3 and from 352 to 1144 μm3, respectively. Nuclear axial ratios were highest in the granular layers. These methods can be used in a variety of comparative ultrastructural studies of epithelia, and will also prove valuable in generating additional biological information from more conventionally presented stereological data.
Similar content being viewed by others
References
Klein-Szanto AJP, Bánóczy J, Schroeder HE (1976) Metaplastic conversion of the differentiation pattern in oral epithelia affected by leukoplakia simplex. A stereologic study. Pathol Eur 11:189–210
Klein-Szanto AJP (1977) Stereologic baseline data of normal human epidermis. J Invest Dermatol 68:73–78
Schroeder HE, Münzel-Pedrazzoli S (1970) Application of stereologic methods to stratified gingival epithelia. J Microsc 92:179–198
Schroeder HE, Münzel-Pedrazzoli S (1970) Morphometric analysis comparing junctional and oral epithelium of normal human gingiva. Helv Odontol Acta 14:53–66
Bernimoulin JP, Schroeder HE (1977) Quantitative electron microscopic analysis of the epithelium of normal human alveolar mucosa. Cell Tissue Res 180:383–401
Meyer M, Schroeder HE (1975) Quantitative electron microscopic analysis of the keratinising epithelium of normal human hard palate. Cell Tissue Res 158:177–203
Landay MA, Schroeder HE (1977) Quantitative electron microscopic analysis of the stratified squamous epithelium of normal human buccal mucosa. Cell Tissue Res 177:383–405
Rowden G (1975) Ultrastructural studies of keratinised epithelia of the mouse. III. Determination of the volumes of nuclei and cytoplasm in murine epidermis. J Invest Dermatol 64:1–3
Rowden G (1975) Ultrastructural studies of keratinised epithelia of the mouse. IV. Quantitative studies of lysosomes. J Invest Dermatol 64:4–8
Andersen L (1978) Quantitative analysis of epithelial changes during wound healing in palatal mucosa of guinea pigs. Cell Tissue Res 193:231–246
Andersen L, Schroeder HE (1978) Quantitative analysis of normal palatal mucosa in guinea pigs. Cell Tissue Res 190:223–233
Franklin CD, Craig GT (1978) Stereological quantification of histological parameters in normal hamster cheek pouch epithelium. Arch Oral Biol 23:337–345
Franklin CD, Craig GT (1978) Stereological quantification of histological parameters in turpentine-induced hyperplasia of hamster cheek pouch epithelium. Arch Oral Biol 23:347–358
Meyer J, Alvares OF, Barrington EP (1970) Volume and dry weight of cells in the epithelium of rat cheek and palate. Growth 34:57–73
Franklin CD, Smith CJ (1980) Stereological analysis of histological parameters in experimental premalignant hamster cheek pouch epithelium. J Pathol 130:201–215
White FH, Mayhew TM, Gohari K (1980) The application of morphometric methods to investigations of normal and pathological stratified squamous epithelium. Pathol Res Pract 166:323–346
Franklin CD, Gohari K, Smith CJ, White FH (1980) Quantitative evaluation of normal, hyperplastic and premalignant epithelium by stereological methods. In: Mackenzie IC, Dabelsteen E, Squier CA (eds) Oral premalignancy: Proceedings of the first Dows Symposium. University of Iowa Press, pp 246–261
Squier CA, Waterhouse JP, Kraucunas E (1973) The application of stereological methods for studying the effects of differing fixative osmolalities on the intercellular space of oral epithelium. I. Normal epithelium. J Oral Pathol 2:127–135
Squier CA, Waterhouse JP, Kraucunas E (1973) The application of stereological methods for studying the effects of differing fixative osmolalities on the intercellular space of oral epithelium. II. Inflamed epithelium. J Oral Pathol 2:136–141
White FH, Gohari K (1981) Variations in the nuclear cytoplasmic ratio during epithelial differentiation in experimental oral carcinogenesis. J Oral Pathol 10:164–172
White FH, Gohari K (1981) Quantitative studies of hemidesmosomes during progressive DMBA carcinogenesis induced in hamster cheek pouch mucosa. Br J Cancer 44:440–450
White FH, Gohari K (1981) A quantitative study of lamina densa alterations in hamster cheek pouch carcinogenesis. J Pathol 135:277–294
Weibel ER (1969) Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol 26:235–302
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137A-138A
White FH, Gohari K (1981) The ultrastructural morphology of hamster cheek pouch epithelium. Arch Oral Biol 26:563–576
Abercrombie M (1946) Estimation of nuclear populations from microtome sections. Anat Rec. 94:239–247
Maser MD, Powell TE, Philpott CE (1967) Relationship among pH, osmolality and concentration of fixative solution. Stain Technol 42:175–182
Pentilla A, Kalimo H, Trump BF (1974) Influence of glutaraldehyde and/or osmium tetroxide on cell volume, ion content, mechanical stability and membrane permeability of Ehrlich ascites tumour cells. J Cell Biol 63:197–214
Pentilla A, McDowell EM, Trump BF (1975) Effects of fixation and postfixation treatments on volume of injured cells. J Histochem Cytochem 23:251–270
Kushida H (1962) A study of cellular swelling and shrinkage during fixation, dehydration and embedding in various standard media. J Electron Microsc (Tokyo) 11:135–142
Tooze J (1964) Measurements of some cellular changes during the fixation of amphibian erythrocytes with osmium tetroxide solutions. J Cell Biol 22:551–563
Elze C, Hennig A (1956) Die inspiratorische Vergrösserung von Volumen und innerer Oberfläche der menschlichen Lunge. Z Anat Entwicklungsgesch 119:457–469
Mayhew TM, Momoh CK (1973) Contribution of the quantitative analysis of neuronal parameters: the effects of biased sampling procedures on estimates of neuronal volume, surface area and packing density. J Comp Neurol 148:217–228
Cope GH, Williams MA (1973) Exocrine secretion in the parotid gland: a stereological analysis at the electron microscopic level of the zymogen granule content before and after isoprenaline-induced degranulation. J Anat 116:269–284
Loud AV (1968) A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol 37:27–46
Cope GH (1978) Stereological analysis of the duct system of the rabbit parotid gland. J Anat 126:591–604
Mayhew TM, Burgess AJ, Gregory CD, Atkinson ME (1979) On the problem of counting and sizing mitochondria: A general reappraisal based on ultrastructural studies of mammalian lymphocytes. Cell Tissue Res 204:297–303
Al-Hamdani MM, Atkinson ME, Mayhew TM (1979) Ultrastructural morphometry of blastogenesis. I. Transformation of small lymphocytes stimulated in vivo with dinitrochlorobenzene. Cell Tissue Res 200:495–509
Albright JT, Listgarten MA (1962) Observations on the fine structure of the hamster cheek pouch epithelium. Arch Oral Biol 7:613–620
Underwood EE (1970) Quantitative stereology. Addison-Wesley Publishing Co Inc, Massachusetts
Kucias J (1970) Estimation of cell metabolism of aquatic animals by microscope measurements. Pol Arch Hydrobiol 17:283–288
Osmanski CP (1971) Disintegrative processes in regions of the oral epithelium. In: Squier CA, Meyer J (eds) Current concepts of the histology of oral mucosa. Charles C Thomas, Springfield, Illinois
Cope GH, Williams MA (1973) Quantitative analyses of the constituent membranes of parotid acinar cells and changes evident after induced exocytosis. Z Zellforsch 145:311–330
Rohr HP, Coffey DS, De Clerk DP, Woodtli HP, Bartsch G (1980) The dog prostate under defined hormonal influences: an approach to experimental induced prostatic growth. Pathol Res Pract 166:347–361
Petrzilka GE, Graf de Beer M, Schroeder HE (1978) Stereological model system for free cells and base line data for human peripheral blood-derived small T-lymphocytes. Cell Tissue Res 192:121–142
Jones AL, Schmucker DL, Mooney JS, Adler RD, Ockner RK (1978) A quantitative analysis of hepatic ultrastructure in rats during enhanced bile secretion. Anat Rec 192:277–288
Reith A, Barnard T, Rohr HP (1976) Stereology of cellular reaction patterns. CRC Crit Rev Toxicol 4:219–269
Author information
Authors and Affiliations
Additional information
A preliminary report of this work has been presented to the Anatomical Society of Great Britain and Ireland and has been published as an abstract [J Anat (1978) 126:409]
Rights and permissions
About this article
Cite this article
White, F.H., Gohari, K. Cellular and nuclear volumetric alterations during differentiation of normal hamster cheek pouch epithelium. Arch Dermatol Res 273, 307–318 (1982). https://doi.org/10.1007/BF00409260
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00409260