Abstract
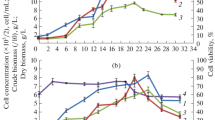
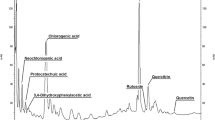
A peanut (Arachis hypogaea L.) cell-suspension culture susceptible to selective induction of stilbene formation was established. The principles of defense responses of the whole plant were found to be retained in the artificial system. The suspension culture was characterized by its growth curve and by various biochemical parameters. In the stationary phase, reached 8 d after transfer to a new medium, the formation of stilbenes and stilbene synthase could be induced without altering the levels of other enzymes. Eighteen hours after applying an artificial elicitor (ultraviolet-C light) or 4 h after eliciting with a crude preparation of Phytophthora cambivora cell walls, phenylalanineammonia-lyase activity was increased eightfold and stilbene-synthase activity 20-fold. The activity of phenylalanine ammonia-lyase reached its peak at a slightly different time from that of stilbene synthase. The main products of L-phenylalanine metabolism in the induced cells were resveratrol, 3,3′,5-trihydroxy-4′-methoxystilbene and isopentenylresveratrol. Likewise, feruloyl-CoA reductase, as a parameter of lignin formation, was enhanced following induction, albeit with a different time course and with a less steep increase than found for phenylalanine ammonia-lyase and stilbene synthase.
Similar content being viewed by others
References
Agrawal, K.M.L., Bahl, O.P. (1972) α-Galactosidase, β-galactosidase, β-glucosidase, β-N-acetylglucosaminidase, and α-mannosidase from pinto beans (Phaseolus vulgaris). Methods Enzymol. 28, 720–728
Bergmeyer, H.U. (1970) Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim
Booth, C. (1971) Fungal culture media. Methods Microbiol. 4, 49–92
Chance, B., Maehly, A.C. (1955) Assay of catalases and peroxidases. Methods Enzymol. 2, 764–775
Coggon, P., Janes, N.F., King, F.E., King, T.J., Molyneux, R.J., Morgan, J.W., Sellars, K. (1965) Hopeaphenol, an extractive of the heartwood of Hopea odorata and Balanocarpus heimii. J. Chem. Soc. 1965, 406–409
Day, D.A., Jenkins, C.L.D., Hatch, M.D. (1981) Isolation and properties of functional mesophyll protoplasts and chloroplasts from Zea mays. Aust. J. Plant Physiol. 8, 21–29
Fritzemeier, K.H., Kindl, H. (1981) Coordinate induction by UV-light of stilbene synthase, phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in leaves of Vitaceae. Planta 151, 48–52
Fritzemeier, K.H., Kindl, H. (1983) 9,10-Dihydrophenanthrenes as phytoalexins of Orchidaceae. Biosynthetic studies in vivo and in vitro proving the route from L-phenylalanine to dihydro-m-coumaric acid, dihydrostilbene and dihydrophenanthrenes. Eur. J. Biochem. 133, 545–550
Fritzemeier, K.H., Rolfs, C.-H., Pfau, J., Kindl, H. (1983) Action of ultraviolet C on stilbene formation in callus of Arachis hypogaea. Planta 159, 25–29
Gamborg, O.L., Miller, R.A., Ojima, K. (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50, 151–158
Hahlbrock, K., Grisebach, H. (1979) Enzymic controls in the biosynthesis of lignin and flavonoids. Annu. Rev. Plant Physiol. 30, 105–130
Hillis, W.E., Hart, J.H., Yazaki, Y. (1974) Polyphenols of Eucalyptus sideroxylon wood. Phytochemistry 13, 1591–1595
Keen, N.T., Inham, J.L. (1976) New stilbene phytoalexins from American cultivars of Arachis hypogaea. Phytochemistry 15, 1794–1795
Kindl, H. (1970) The regulation of the L-tyrosine ammonialyase activity by phenolic compounds. Hoppe-Seyler's Z. Physiol. Chem. 351, 792–798
Kindl, H. (1985) Biosynthesis of stilbenes. In: Biosynthesis and biodegradation of wood compounds, pp. 349–377, Higuchi, T., ed. Academic Press, New York
Kindl, H. (1986) Stilbenes and dihydrophenanthrenes as phytoalexins. Synthesis and control. Biol. Chem. Hoppe-Seyler 367, 75
Kossatz, V.C., van Huystee, R.B. (1976) The specific activities of peroxidase and aminolevulinic acid dehydratase during the growth cycle of peanut suspension culture. Can. J. Bot. 54, 2089–2094
Langcake, P., Pryce, R.J. (1977) The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry 16, 1193–1196
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275
Postius, C., Kindl, H. (1978) The occurrence of phenylalanine ammonia-lyase and cinnamic acid p-hydroxylase on the endoplasmic reticulum of cell suspension cultures of Glycine max. Z. Naturforsch. 33c, 65–69
Rolfs, C.H., Fritzemeier, K.H., Kindl, H. (1981) Cultured cells of Arachis hypogaea susceptible to induction of stilbene synthase (Resveratrol forming). Plant Cell Reports 1, 83–85
Schenk, R.U., Hildebrandt, A.C. (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50, 199–204
Schöppner, A., Kindl, H. (1979) Stilbene synthase (Pinosylvin synthase) and its induction by UV-light. FEBS Lett. 108, 349–352
Sols, A., Salas, M.L. (1966) Phosphofructokinase, III. Yeast. Methods Enzymol. 9, 436–443
Stephan, D., van Huystee, R.B. (1981) Some aspects of peroxidase synthesis by cultured peanut cells. Z. Pflanzenphysiol. 101, 313–321
Wengenmayer, H., Ebel, J., Grisebach, H. (1976) Enzymic synthesis of lignin precursors. Purification and properties of a cinnamoyl-CoA: NADPH reductase from cell suspension cultures of soybean (Glycine max). Eur. J. Biochem. 65, 529–536
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rolfs, C.H., Schön, H., Steffens, M. et al. Cell-suspension culture of Arachis hypogaea L.: model system of specific enzyme induction in secondary metabolism. Planta 172, 238–244 (1987). https://doi.org/10.1007/BF00394593
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00394593