Summary
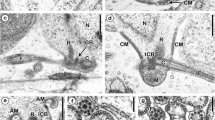
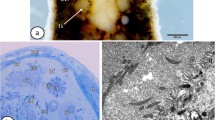
Spermatogenesis in Polytrichum juniperrinum includes a series of precise and coordinated morphogenetic movements among the organelles of the androcyte. The basal bodies, the underlying microtubules, and the multilayered structure (MLS) are positioned as an integrated unit at the periphery of the cell, and the nucleus migrates into contact with them. The shape of the nucleus begins to change, with the formation of an anterior projection, or beak. The mitochondrial sheath that has coalesced on the plastid divides to form the apical body, and elongation of the nucleus begins. The posterior basal body and one microtubule undergo lateral displacement from the rear forward, as elongation of the nucleus and the microtubules continues. The mitochondrial mass that is now the apical body grows rearward along the side of the elongated nucleus, and the two groups of microtubules in the MLS rearrange themselves. The lower elements of the MLS also participate in the morphogenetic rearrangement. By the time the nucleus has elongated once around the cell and the apical body has begun it s rearward growth, the lower elements of the MLS are found only beneath the anterior basal body. Subsequently these layers disappear from this location also.
Similar content being viewed by others
References
Allen, C. E.: The spermatogenesis of Polytrichum juniperinum. Ann. Bot. 31 269–292 (1917).
Carothers, Z. B., and G. L. Kreitner: Studies of spermatogenesis in the Hepaticae. I. Ultrastructure of the Vierergruppe in Marchantia. J. Cell Biol. 33, 43–51 (1967).
Diers, L.: Der Feinbau des Spermatozoids von Sphaerocarpos donnellii Aust. (Hepaticae). Planta (Berl.) 72, 119–145 (1967).
Eyme, J.: Recherches cytologiques sur les mousses. Botaniste 38, 1–166 (1954).
Gibbons, I. R.: Structural asymmetry in cilia and flagella. Nature (Lond.) 190, 1128–1129 (1961).
—: A method for obtaining serial sections of known orientation from single spermatozoa. J. Cell Biol. 16, 626–629 (1963).
— and A. V. Grimstone: On flagellar structure in certain flagellates. J. biophys. biochem. Cytol. 7, 697–716 (1960).
Heitz, E.: Elektronenmikroskopische Untersuchungen über zwei auffallende Strukturen an der Geisselbasis der Spermatiden von Marchantia polymorpha, Preissia quadrate, Sphaerocarpus Donnellii, Pellia Fabroniana (Hepaticae). Z. Naturforsch. 14b, 399–401 (1959).
Heitz, E.: Über die Geisselstruktur sowie die Dreiergruppe in den Spermatiden der Leberund Laubmoose. Proc. Europ. Region. Conf. Electron Microcsopy, Delft 1960, vol. 2, p. 934–937.
Ledbetter, M. C., and K. R. Porter: A “mictotubule” in plant cell fine ultrastructure. J. Cell Biol. 19, 239–250 (1963).
Manton, I.: The possible significance of some details of flagellar bases in plants. J. roy. micr. Soc. 82, 279–285 (1964).
Mizukami, I., and J. Gall: Centriole replication. II. Sperm formation in the fern, Marsilea, and the cycad, Zamia. J. Cell Biol. 29, 97–111 (1966).
Mollenhauer, H. H.: Plastic embedding mixtures for use in electron microscopy. Stain Technol. 29, 111–114 (1964).
Motte, J.: Contribution à la connaissance cytologique des muscinées. Ann. Sci. natur. Bot., Sér. X, 10, 293–543 (1928).
Paolillo Jr, D. J.: On the androcyte of Polytrichum, with special reference to the Dreiergruppe and the limosphere (Nebenkern). Canad. J. Bot. 43, 669–676 (1965).
Reynolds, E. S.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963).
Ringo, D. L.: Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543–571 (1967).
Satir, P.: Structure and function in cilia and flagella. Facts and problems. In: Protoplasmatologia No. 3E. Vienna: Springer 1965.
Sato, S.: The filamentous appendage in the spermatozoids of Hepaticae as revealed by the electron microscope. Bot. Mag. (Tokyo) 69, 435–438 (1956).
Stempak, J. G., and R. T. Ward: An improved staining method for electron microscopy. J. Cell Biol. 22, 697–701 (1964).
Weier, T. E.: A study of the moss plastid after fixation by mitochondrial, osmium and silver techniques. II. The plastid during spermatogenesis in Polytrichum commune and Catharinaea undulata. Cellule 41, 51–84 (1931).
Wilson, M.: Spermatogenesis in the Bryophyta. Ann. Bot. 25, 415–459 (1911).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paolillo, D.J., Kreitner, G.L. & Reighard, J.A. Spermatogenesis in Polytrichum juniperinum . Planta 78, 226–247 (1968). https://doi.org/10.1007/BF00386424
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00386424