Summary
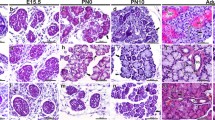
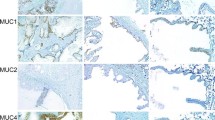
The distribution of selective cytokeratin polypeptides, vimentin, and glial fibrillary protein (GFP) in 5 human cystadenolymphomas of the parotid gland was compared with normal human parotid (n=5) and submandibular (n=4) glands using a panel of monoclonal antibodies against diverse and selective cytokeratin polypeptides, vimentin and glial fibrillary protein (GFP). A biotin-streptavidin method was used on cryostat sections. The immunocytochemical finding of identical cytokeratin polypeptides Nos. 7, 8, 18 and 19 and basal cells selectively labeled by the monoclonal antibody KS 8.58, in both the epithelial part of the cystadenolymphomas and in the duct epithelium of the parotid gland, confirms the hypothesis that the epithelial compartment of cystadenolymphomas is derived from the duct system. The triple expression of cytokeratin, vimentin and GFP in myoepithelial cells of the parotid gland is discussed.
Similar content being viewed by others
References
Achtstätter T, Moll R, Anderson A, Kuhn C, Pilz S, Schwechheimer K, Franke WW (1986) Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenoma. Differentiation 31:206–227
Born IA, Schwechheimer K, Maier H, Möller P, Otto HF (1986) Peanut-Agglutinin als Marker für undifferenzierte Basalzellen (sog. undifferente Gangepithelzellen) in normalem Speicheldrüsengewebe und Cystadenolymphom. Verh Dtsch Ges Pathol 70:463
Budka H (1986) Non-glial specificities of immunocytochemistry for the glial fibrillary acidic protein (GFAP). Triple expression of GFAP, vimentin and cytokeratins in papillary meningioma and metastasizing renal carcinoma. Acta Neuropathol (Berl) 72:43–54
Caselitz J, Osborn M, Seifert G, Weber K (1981) Intermediate sized filament proteins (prekeratin, vimentin, desmin) in normal parotid gland and parotid gland tumors. Virchows Arch [A] 393:273–286
Caselitz J, Osborn M, Wustrow J, Seifert G, Weber K (1982a) The expression of different intermediate sized filaments in human salivary glands and their tumors. Pathol Res Pract 175:266–278
Caselitz J, Seifert G, Jaup T (1982b) Tumor antigens in neoplasms of the parotid gland. J Oral Pathol 11:374–386
Caselitz J, Salfelder G, Seifert G (1984) Adenolymphoma: an immunohistochemical study with monoclonal antibodies against lymphocyte antigens. J Oral Pathol 13:438–447
Debus E, Weber K, Osborn M (1982) Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J 1:1641–1647
Debus E, Weber K, Osborn M (1983) Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation 25:193–203
Franke WW, Schmid E, Freudenstein C, Appelhans B, Osborn M, Weber K, Keenan TW (1980) Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol 84:633–654
Geiger S, Geiger B, Leitner O, Marshak G (1987) Cytokeratin polypeptides expression in different epithelial elements of human salivary glands. Virchows Arch [A] 410:403–414
Graham RC, Lundholm U, Karnovsky MJ (1965) Cytochemical demonstration of peroxidase activity with 3-amino-9-ethylcarbazole. J Histochem Cytochem 13:150–152
Holthöfer H, Miettinen A, Lehto V-P, Linder E, Alfthan O, Virtanen I (1983) Cellular origin and differentiation of renal cell carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest 50:552–559
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison of ABC and unlabeled antibody (PAP) procedure. J Histochem Cytochem 30:577–580
Krepler R, Denk H, Artlieb U, Moll R (1982) Immunocytochemistry of intermediate filament proteins present in pleomorphic adenomas of the human parotid gland. Characterization of different cell types in the same tumor. Differentiation 21:191–199
Lane BE (1982) Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol 92:665–673
Lane BE, Bartek J, Purkis PE, Leigh MJ (1985) Keratin antigens in differentiating skin. Ann NY Acad Sci USA 455:241–258
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors, and cultured cells. Cell 31:11–24
Moll R (1986) Epitheliale Tumormarker. Verh Dtsch Ges Pathol 70:28–50
Nakazato Y, Ishizeki J, Takahashi K, Yamaguchi H, Kamei T, Mori T (1982) Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenomas of the salivary glands. Lab Invest 46:621–626
Nakazato Y, Ishida Y, Takahashi K, Suzuki K (1985) Immunohistochemical distribution of S-100 protein and glial fibrillary acidic proteins in normal and neoplastic salivary glands. Virchows Arch [A] 405:299–310
Osborn M, Geisler N, Shaw G, Sharp G, Weber K (1982) Intermediate filaments. Cold Spring Harbor Symp Quant Biol 46:413–429
Osborn M, Altmannsberger M, Debus E, Weber K (1985) Differentiation of the maior human tumor groups using conventional and monoclonal antibodies specific for individual intermediate filament proteins. Ann NY Acad Sci USA 455:649–668
Osborn M, Debus E, Weber K (1984) Monoclonal antibodies specific for vimentin. Eur J Cell Biol 34:137–143
Ramaekers FCS, Huysmans A, Moesker O, Kant A, Jap PHK, Herman CJ, Vooijs GP 1983) Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumours. Use in surgical pathology. Lab Invest 49:353–361
Seifert G (1984) Der Einsatz von Tumormarkern bei der Diagnostik von Speicheldrüsentumoren. Wien Klin Wochenschr 94:372–375
Seifert G, Miehlke H, Haubrich J, Chilla R (Hrsg) (1984) Speicheldrüsenkrankheiten. Pathologie-Klinik-Therapie-Fazialischirurgie. Thieme, Stuttgart New York
Seifert G (1985) The importance of tumor markers in oral pathology. II. Cell membrane and cytoplasmic antigens as tumour markers. Pathol Res Pract 179:625–628
Schwechheimer K (1986) Nervale Tumormarker. Verh Dtsch Ges Pathol 70:82–103
Thompson AS, Bryant HC (1950) Histogenesis of papillary crystadenoma lymphomatosum (WARTHINs tumor) of the parotid salivary gland. Am J Pathol 26:807–827
Tölle H-G, Weber K, Osborn M (1985) Microinjection of monoclonal antibodies specific for one intermediate filament in cells containing multiple keratins allow insight into the composition of particular 10 nm filaments. Eur J Cell Biol 38:234–244
van Muijen GNP, Ruiter DJ, Franke WW, Achtstätter T, Haasnoot W, Ponec M, Warnaar SO (1986) Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res 162:97–113
Viac J, Reano A, Brochier J, Staquet M-J, Thivolet J (1983) Reactivity pattern of a monoclonal antikeratin antibody (KL 1). J Invest Dermatol 81:351–354
Woodcock-Mitchell J, Eichner R, Nelson WG, Sun T-T (1982) Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol 95:580–588
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Born, I.A., Schwechheimer, K., Maier, H. et al. Cytokeratin expression in normal salivary glands and in cystadenolymphomas demonstrated by monoclonal antibodies against selective cytokeratin polypeptides. Vichows Archiv A Pathol Anat 411, 583–589 (1987). https://doi.org/10.1007/BF00713290
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00713290