Summary
The central projections of primary afferent fibers of the greater splanchnic nerve of the rat were investigated using the transganglionic horseradish peroxidase transport technique. In addition, the corresponding spinal ganglion cells and the preganglionic sympathetic neurons were demonstrated. For comparing visceral and somatic afferents, intercostal nerve afferents were labelled by the same technique.
Splanchnic afferent dorsal root ganglion cells were found at segments T3 to T13 ipsilaterally, with the greatest density at T8 to T12. Labelled cells represented about 10%–15% of all neurons in the ganglia at maximal projection levels. They were randomly distributed within individual ganglia. The great majority were medium to small sized and round to slightly oval in shape.
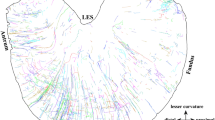
In the spinal cord, labelled visceral afferent axons were found maximally at T8 to T11, but could be detected in decreasing density up to T1 and down to L1. They were distributed over Lissauer's tract and the dorsal funiculus to a medial and lateral collateral pathway (MCP and LCP, respectively). The MCP, somewhat more prominent than the LCP, was destined primarily to clustered presumptive terminal fields in medial lamina I and outermost lamina IIa. Only a few axons continued further to laminae V and X. Splanchnic afferent axons, most likely derived from the MCP, formed a longitudinal bundle ventral to the central canal. The LCP consisted of more or less well-defined axon bundles emanating from the lateral Lissauer's tract and curving round the lateral edge of the dorsal horn and through the dorsolateral funiculus. Presumptive terminal sites of LCP axons are the lateral laminae I and IIa, the nucleus of the dorsolateral funiculus and the dorsal part of lamina V. A few LCP axons were seen in the vicinity of lateral dendrites of preganglionic sympathetic axons. Visceroafferent terminals were absent from laminae IIb–IV and VII. The possible consequences of the MCP/LCP duality for the central connections of splanchnic afferents are discussed. Some splanchnic afferents ascended to the gracile and cuneate nuclei, and rarely to the spinal trigeminal nucleus.
These results fit into the general concept of visceroafferent terminal organization that has emerged during the last few years. Differences to other reports in the detailed arrangement of fibers and terminals are discussed.
Somatoafferent cell bodies represented the vast majority of neurons in the respective spinal ganglia. Cell sizes encompassed the whole range from very small to very large without a clear predominance of one particular size class. Cell shapes of somatic neurons were more variable than those of visceral afferent neurons. Somatic afferent fibers and presumptive terminals in the spinal cord are distributed ipsilaterally to dorsal horn laminae I–V, most heavily II–IV, to the nucleus dorsalis Clarke, to the ventral horn, and also sparsely to the dorsal horn contralaterally.
Labelled preganglionic sympathetic neurons were found in segments T3–T13. The vast majority was located in the intermediolateral nucleus. Fewer neurons occurred in the intercalated nucleus, and occasionally a neuron was labelled in the dorsal grey commissure.
Similar content being viewed by others
References
Abrahams VC, Richmond FJ, Keane J (1984) Projections from C2 and C3 nerves supplying muscles and skin of the cat neck: A study using transganglionic transport of horseradish peroxidase. J Comp Neurol 230:142–154
Aidar O, Geohegan WA, Ungewitter LH (1952) Splanchnic afferent pathways in the central nervous system. J Neurophysiol 15:131–138
Alvarez WC (1931) Abdominal pain. Am J Surg 14:385–394
Amassian VE (1951) Fiber groups and spinal pathways of cortically represented visceral afferents. J Neurophysiol 14:445–460
Ammons WS, Foreman RD (1984) Responses of T2–T4 spinal neurons to stimulation of the greater splanchnic nerves of the cat. Exp Neurol 83:288–301
Ammons WS, Blair RW, Foreman RD (1984) Greater splanchnic excitation of primate T1–T5 spinothalamic neurons. J Neurophysiol 51:592–603
Andres KH (1961) Untersuchungen über den Feinbau von Spinalganglien. Z Zellforsch 55:1–48
Bain WA, Irving JT, McSwiney BA (1935) The afferent fibers from the abdomen in the splanchnic nerves. J Physiol (Lond) 84:323–333
Barja F, Mathison R (1984) Sensory innervation of the rat portal vein and the hepatic artery. J Autonom Nerv Syst 10:117–125
Bresnahan JC, Ho RH, Beattie MS (1984) A comparison of the ultrastructure of substance P and enkephalin-immunoreactive elements in the nucleus of the dorsal lateral funiculus and laminae I and II of the rat spinal cord. J Comp Neurol 229:497–511
Brown AG (1981) Organization in the spinal cord. Springer, Berlin Heidelberg New York
Calaresu FR, Faiers AA, Mogenson GJ (1976) Central neural regulation of heart and blood vessels in mammals. In: Kerkut GA, Phillis JW (eds) Progress in neurobiology, vol 5, pt 1. Pergamon Press, Oxford, pp 1–35
Calvino B, Villanueva L, LeBars D (1984) The heterotopic effects of visceral pain: Behavioural and electrophysiological approaches in the rat. Pain 20:261–271
Carpenter MB, Stein BM, Shriver JE (1968) Central projections of spinal dorsal roots in the monkey. II. Lower thoracic, lumbosacral and coccygeal dorsal roots. Am J Anat 123:75–118
Caverson MM, Ciriello J, Calaresu FR (1983) Direct pathway from cardiovascular neurons in the ventrolateral medulla to the region of the intermediolateral nucleus of the upper thoracic cord: an anatomical and electrophysiological investigation in the cat. J Autonom Nerv Syst 9:451–475
Cervero F (1983a) Somatic and visceral inputs to the thoracic spinal cord of the cat: Effects of noxious stimulation of the biliary system. J Physiol (Lond) 337:51–67
Cervero F (1983b) Supraspinal connections of neurones in the thoracic spinal cord of the cat: Ascending projections and effects of descending impulses. Brain Res 275:251–261
Cervero F (1985) Visceral nocieption: Peripheral and central aspects of visceral nociceptive systems. Phil Trans R Soc Lond B 308:325–337
Cervero F, Connell LA (1984a) Fine afferent fibers from viscera do not terminate in the substantia gelatinosa of the thoracic spinal cord. Brain Res 294:370–374
Cervero F, Connell LA (1984b) Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol 230:88–98
Cervero F, McRitchie (1982) Neonatal capsaicin does not affect unmyelinated efferent fibers of the autonomic nervous system: Functional evidence. Brain Res 239:283–288
Cervero F, Connell LA, Lawson SN (1984) Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J Comp Neurol 228:422–431
Changgeng Z, Zenker W, Celio M (1983) Substance P-positive structures in rat spinal cord. A longitudinal bundle ventral to the central canal. Anat Anz (Jena) 154:193–203
Chung K, Langford LA, Applebaum AE, Coggeshall RE (1979) Primary fibers in the tract of Lissauer in the rat. J Comp Neurol 184:587–598
Ciriello J, Calaresu FR (1983) Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Autonom Nerv Syst 8:273–285
Corner MA, Veltman WAM, Baker RE, van de Nes J (1978) Topography of cutaneous spinal ganglion cells in the frog (Rana esculenta). Brain Res 156:151–156
Craig AD, Mense S (1983) The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett 41:233–238
Crousillat J, Ranieri F, Perrin J (1979) Interactions entre afférences somatiques et viscérles le long de la voie sensitive splanchnicque. Arch Ital Biol 117:186–188
Dalsgaard C-J, Lundberg JM (1984) Evidence for a spinal afferent innervation of the guinea pig lower respiratory tract as studied by the horseradish peroxidase technique. Neurosci Lett 45:117–122
Dietrichs E, Walberg F (1979) The cerebellar projection from the lateral reticular nucleus as studied with retrograde transport of horseradish peroxidase. Anat Embryol 155:273–290
Dodd J, Jessell TM (1984) Monoclonal antibodies that identify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn. Pain Suppl 2:S143
Donovan MK, Wyss, JM, Winternitz SR (1983) Localization of renal sensory neurons using the fluorescent dye technique. Brain Res 259:119–122
Duce IR, Keen P (1977) An ultrastructural classification of the neuronal cell bodies of the rat dorsal root ganglion using zinc iodate-osmium impregnation. Cell Tissue Res 185:263–277
Elfvin LG, Lindh B (1982) A study of the extrinsic innervation of the guinea pig pylorus with the horseradish peroxidase tracing technique. J Comp Neurol 208:317–324
Fitzgerald M (1983), Capsaicin and sensory neurones-a review. Pain 15:109–130
Foreman RD, Hancock MB, Willis WD (1981) Responses of spinothalamic tract cells in the thoracic spinal cord of the monkey to cutaneous and visceral inputs. Pain 11:149–162
Foreman RD, Blair RW, Weber RN (1984) Viscerosomatic convergence onto T2–T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic tract neurons in the cat. Exp Neurol 85:597–619
Gabella G (1976) Structure of the autonomic nervous system. Chapman and Hall, London
Gammon GD, Bronk DW (1935) The discharge of impulses from pacinian corpuscles in the mesentery and its relation to vascular changes. Am J Physiol 114:77–84
Gernandt B, Zotterman Y (1946) Intestinal pain: An electrophysiological investigation on mesenteric nerves. Acta Physiol Scand 12:56–72
Giesler, JR GJ Elde RP (1984) Immunocytochemical studies of the lateral cervical and lateral spinal nuclei. Pain Suppl 2:S130
Giesler Jr GJ, Menétrey D, Basbaum AI (1979) Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol 184:107–126
Gobel S (1979) Neural circuitry in the substantia gelatinosa of Rolando: Anatomical insights. In: Bonica JJ, Liebeskind JC, Albe-Fessard DG (eds) Adv Pain Res Ther, vol 3. Raven Press, New York, pp 175–195
Gobel S, Falls WM (1979) Anatomical observations of horseradish peroxidase-filled terminal primary axonal arborizations in layer II of the substantia gelatinosa of Rolando. Brain Res 175:335–340
Gobel S, Falls WM, Humphrey E (1981) Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: candidates for the terminal axonal arbors of primary neurons with unmyelinated (C) axons. J Neurosci 1:1163–1179
Gokin P, Kostyuk P-G, Preobrazhensky N-N (1977) Neuronal mechanisms of interactions of high-threshold visceral and somatic afferent influences in spinal cord and medulla. J Physiol (Paris) 73:319–333
Goodman CS, Bastiani MJ, Doe CQ, duLac S, Helfand SL, Kuwada JY, Thomas JB (1984) Cell recognition during neuronal development. Science 225:1271–1279
Groat WC de, Nadelhaft I, Morgan C, Schauble T (1978) Horseradish peroxidase tracing of visceral efferent and primary afferent pathways in the cat's sacral spinal cord using benzidine processing. Neurosci Lett 10:103–108
Groat WC de, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K (1981) Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Autonom Nerv Syst 3:135–160
Gwyn DG, Waldron HA (1968) A nucleus in the dorsolateral funiculus of the spinal cord of the rat. Brain Res 10:342–351
Hamer DW, Santer RM (1981) Anatomy and blood supply of the coeliac-superior mesenteric ganglion complex of the rat. Anat Embryol 162:353–362
Hancock and Peveto (1979) A preganglionic autonomic nucleus in the dorsal gray commissure of the lumbar spinal cord of the rat. J Comp Neurol 183:65–72
Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol (Lond) 359:31–46
Head H (1893) On distubances of sensation with especial reference to the pain of visceral disease. Brain 16:1–133
Hino O, Kanafusa K, Tsunekawa K (1979) Quantitative histological study of spinal afferent innervation on the ventral surface of the cat stomach by horseradish peroxidase (HRP) method. Experientia 35:379–380
Hökfelt T, Johansson O, Kellerth J-O, Ljungdahl Å, Nilsson G, Nygårds A, Pernow B (1977) Immunohistochemical distribution of substance P. In: Substance P, von Euler US, Pernow B (eds) Raven Press, New York, pp 117–145
Horch KW, Burgess PR, Whitehorn D (1976) Ascending collaterals of cutaneous neurons in the fasciculus gracilis of the cat. Brain Res 117:1–17
Hrycyshyn AW, Flumerfelt BA, Anderson WA (1982) A horseradish peroxidase study of the projections from the lateral reticular nucleus to the cerebellum in the rat. Anat Embryol 165:1–18
Illing R-B, Wässle H (1979) Visualization of the HRP reaction product using the polarization microscope. Neurosci Lett 13:7–11
Jancsó G, Hökfelt T, Lundberg JM, Kiraly E, Halász N, Nilsson G, Terenius L, Rehfeld J, Steinbusch H, Verhofstad A, Elde R, Said S, Brown M (1981) Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/ CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol 10:963–980
Kalia M, Sullivan JM (1982) Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211:248–264
Kubik I, Szabo J (1955) Die Innervation der Lymphgefässe im Mesenterium. Acta Morphol Acad Sci Hung 6:25–31
Kuo DC, deGroat WC (1985) Primary afferent projections of the major splanchnic nerve to the spinal cord and gracile nucleus of the cat. J Comp Neurol 231:421–434
Kuo DC, Yamasaki DS, Krauthamer GM (1980) Segmental organization of sympathetic preganglionic neurons of the splanchnic nerve as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett 17:11–16
Kuo DC, Krauthamer GM, Yamasaki DS (1981) The organization of visceral sensory neurons in thoracic dorsal root ganglia (DRG) of the cat studied by horseradish peroxidase (HRP) reaction using the cryostat. Brain Res 208:187–191
Kuo DC, Yang GCH, Yamasaki DS, Krauthamer GM (1982) A wide field electron microscopic analysis of the fiber constituents of the major splanchnic nerve in cat. J Comp Neurol 210:49–58
Kuo DC, Nadelhaft I, Hisamitsu T, deGroat WC (1983) Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol 216:162–174
Kuo DC, Oravitz JJ, deGroat WC (1984) Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res 321:111–118
Lappe RW, Webb RL, Boutelle S, Brody MJ (1982) Identification of central projections of renal afferent nerves. Fed Proc 41:1258
LeBars D, Dickenson AH, Besson J-M (1979) Diffuse noxious inhibitor controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6:283–304
Lebedev VP, Rosanov NN, Skobelev VA, Smirnov KA (1976) Study of the early somatosympathetic reflex response. Neurosci Lett 2:319–323
Lembeck F, Skofitsch G (1982) Visceral pain reflex after pretreatment with capsaicin and morphine. Naunyn-Schmiedeberg's Arch Pharmacol 321:116–122
Li CL, Mathews G, Bak AF (1977) Action potential of somatic and autonomic nerves. Exp Neurol 56:527–537
Li G, Fan Y, Wang J (1983) Tracing the origin of the sympathetic afferent nerve of the ventriculus cordis by means of horseradish peroxidase method in the rat. Acta Acad Med Wuhan 3:147–152
Light AR, Perl ER (1979a) Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol 186:117–132
Light AR, Perl ER (1979b) Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186:133–150
Lima D, Coimbra A (1983) The neuronal population of the marginal zone (lamina I) of the rat spinal cord. A study based on reconstructions of serially sectioned cells. Anat Embryol 167:273–288
Liu RPC (1983) Laminar origins of spinal projection neurons to the periaqueductal gray of the rat. Brain Res 264:118–122
Magni F, Carobi C (1983) The afferent and preganglionic parasympathetic innervation of the rat liver, demonstrated by the retrograde transport of horseradish peroxidase. J Autonom Nerv Syst 8:237–260
Mawe GM, Bresnahan JC, Beattie MS (1984) Primary afferent projections from dorsal and ventral roots to autonomic preganglionic neurons in the cat sacral spinal cord: light and electron microscopic observations. Brain Res 290:152–157
McSwiney BA, Suffolk SF (1938) Segmental distribution of certain visceral afferent neurones of the pupillo-dilator reflex in the cat. J Physiol (Lond) 93:104–116
Mei N, Ranieri F, Crousillat J (1970) Limites et distribution des afferences splanchniques au niveau des ganglions spinaux chez le chat. CR Soc Biol Paris 164:1058–1062
Menétrey D, Chaouch A, Besson JM (1980) Location and properties of dorsal horn neurons at origin of spinoreticular tract in lumbar enlargement of the rat. J Neurophysiol 44:862–877
Menétrey D, Chaouch A, Binder D, Besson JM (1982) The origin of the spinomesencephalic tract in the rat: an anatomical study using the retrograde transport of horseradish peroxidase. J Comp Neurol 206:193–207
Menétrey D, Roudier F, Besson JM (1983) Spinal neurons, reaching the lateral reticular nucleus as studies in the rat by retrograde transport of horseradish peroxidase. J Comp Neurol 220:439–452
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Mesulam MM, Hegarty E, Barbas H., Carson KA, Gower EC, Knapp AG, Moss MB, Mufson EJ (1980) Additional factors influencing sensitivity in the tetramethyl benzidine method for horseradish peroxidase neurohistochemistry. J Histochem Cytochem 28:1255–1259
Meyers DER, Snow PJ (1982) The morphology of physiologically identified deep spinothalamic tract cells in the lumbar spinal cord of the cat. J Physiol (Lond) 329:373–388
Molander C, Xu Q, Grant G (1984) The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol 230:133–141
Morgan C, Nadelhaft I, deGroat WC (1981) The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol 201:415–444
Mysicka A, Zenker W (1981) Central projections of muscle afferents from the sternomastoid nerve in the rat. Brain Res 211:257–265
Nadelhaft I, Booth AM (1984) The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol 226:238–245
Nadelhaft I, Roppolo J, Morgan C, deGroat WC (1983) Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. J Compl Neurol 216:36–52
Neuhuber W (1982) The central projections of visceral primary afferent neurons of the inferior mesenteric plexus and hypogastric nerve and the location of the ralated sensory and preganglionic sympathetic cell bodies in the rat. Anat Embryol 164:413–425
Neuhuber W, Neiderle B (1979) Spinal ganlion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP). Anat Embryol 155:355–362
Neuhuber W, Niederle B (1980) Differential labelling by horseradish peroxidase of small and large spinal neurons of rats. Neurosci Lett 20:131–134
Neuhuber W, Sandoz PA (1985) Combination of the glucose oxidase method with silver-gold intensification for demonstrating transganglionic transport of HRP in primary vagal afferents. Experientia 41:775–776
Newman PP (1974) Visceral afferent functions of the nervous system. Arnold, London
Nord SG (1967) Somatotopic organization in the spinal trigeminal nucleus, the dorsal column nuclei and related structures in the rat. J Comp Neurol 130:343–356
Oldfield BJ, McLachlan EM (1978) Localization of sensory neurons traversing the stellate ganglion of the cat. J Comp Neurol 182:915–922
Perrin J, Crousillat J (1983) Splanchnic afferent input to the lateral reticular nucleus of the cat. J Autonom Nerv Syst 8:383–393
Petras JM, Cummings JF (1972) Autonomic neurons in the spinal cord of the rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fiber degeneration following dorsal rhizotomy. J Comp Neurol 146:189–218
Pfister J, Zenker W (1984) The splenius capitis muscle of the rat, architecture and histochemistry, afferent and efferent innervation as compared with that of the quadriceps muscle. Anat Embryol 169:79–89
Pomeranz B, Wall PD, Weber WV (1968) Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol (Lond) 199:511–532
Ranieri F, Mei N, Crousillat J (1973) Les afférences splanchniques provenant des méchanorécepteurs gastro-intstinaux et peritonéaux. Exp Brain Res 16:276–290
Ranieri F, Crousillat J, Mei N (1975) Etude électrophysiologique et histologique des fibres afférentes splanchniques. Arch Ital Biol 113:354–373
Réthelyi M (1974) Spinal transmission of autonomic processes. J Neurol Trans Suppl 11:195–212
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Rigamonti DD, Hancock MB (1978) Viscerosomatic convergence in the dorsal column nuclei of the cat. Exp Neurol 61:337–348
Rigamonti DD, Hancock MB, Kapur SP (1978) Visceral afferent projections to the dorsal column nuclei. Brain Res 150:408–412
Roppolo JR, Nadelhaft I, deGroat WC (1985) The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol 234:475–488
Ruch TC (1947) Visceral sensation and referred pain. In: Fulton JF (ed), Howell's textbook of physiology, 15th edn. Saunders, Philadelphia, pp 385–401
Rucker HK, Holloway, JA, Keyser GF (1984) Response characteristics of cat spinothalamic tract neurons to splanchnic nerve stimulation. Brain Res 291:383–387
Sato A, Schmidt RF (1973) Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev 53:916–947
Selzer M, Spencer WA (1969) Convergence of visceral and cutaneous afferent pathways in the lumbar spinal cord. Brain Res 14:331–348
Sharkey KA, Williams RG (1983) Extrinsic innervation of the rat pancreas: demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett 42:131–135
Sheehan D, (1932) The afferent nerve supply of the mesentery and its significance in the causation of abdominal pain. J Anat 67:233–249
Shriver JE, Stein BM, Carpenter MB (1968) Central projections of spinal dorsal roots in the monkey. I. Cervical and upper thoracic dorsal roots. Am J Anat 123:27–74
Simon OR, Schramm LP (1984) The spinal course and medullary termination of myelinated renal afferents in the rat. Brain Res 290:239–247
Smith CL (1983) The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol 220:29–43
Sommer EW, Kazimierczak J, Droz B (1984) Subclasses of primary sensory neurons in the mouse revealed by ultrastructural features, cytoenzymatic criteria and substance P immunocytochemistry. Neurosci Lett Suppl 18: S252
Torigoe Y, Cernucan RD, Nishimoto JAS, Blanks RHI (1985) Sympathetic preganglionic efferent and afferent neurons mediated by the greater splanchnic nerve in rabbit. Exp Neurol 87:334–348
Ueyama T, Mizuno N, Nomura S, Konishi A, Itoh K, Arakawa H (1984) Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol 222:38–46
Ueyama T, Mizuno N, Takahashi O, Nomura S, Arakowa H, Matsushima R (1985) Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol 232:548–556
Vance WH, Bowker RC (1983), Spinal origins of cardiac afferents from the region of the left anterior descending artery. Brain Res 258:96–100
Waldrop TG, Iwamoto GA, Ordway GA (1984) Cardiovascular reflexes arising from the gallblader and pancreas in spinal and in decerebrate cats. Brain Res 299:358–362
Warrington WB, Griffith F (1904) On the cells of the spinal ganglia and on the relationship of their histological structure to their axonal distribution. Brain 27:297–326
Weaver LC, Fry HK, Meckler RL, Oehl RS (1983) Multisegmental spinal sympathetic reflexes orginating from the heart. Am J Physiol 245:R345-R352
White JC, Sweet WH (1955) Pain. Its mechanisms and neurosurgical control. Thomas, Springfield
Wyss JM, Donovan MK (1984) A direct projection from the kidney to the brainstem. Brain Res 298:130–134
Ygge J (1984) On the organization of the thoracic spinal ganglion and nerve in the rat. Exp Brain Res 55:395–401
Ygge J, Aldskogius H (1984) Intercostal nerve transection and its effect on the dorsal root ganglion. A quantitative study on thoracic ganglion cell numbers and sizes in the rat. Exp Brain Res 55:402–408
Ygge J, Grant G (1983) The organization of the thoracic spinal nerve projection in the rat dorsal horn demonstrated with transganglionic transport of horseradish peroxidase. J Comp Neurol 216:1–9
Ygge J, Aldskogius H, Grant G (1981) Asymmetries and symmetries in the number of thoracic dorsal root ganglion cells. J Comp Neurol 202:365–372
Yoshida S, Matsuda Y (1979) Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidaseinjection techniques. J Neurophysiol 42:1134–1145
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. W. Zenker on occasion of his 60th birthday
Parts of this study have been presented in abstract form at the 8th ENA meeting in Den Haag, September 1984
Rights and permissions
About this article
Cite this article
Neuhuber, W.L., Sandoz, P.A. & Fryscak, T. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. Anat Embryol 174, 123–144 (1986). https://doi.org/10.1007/BF00318344
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318344