Summary
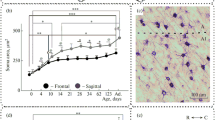
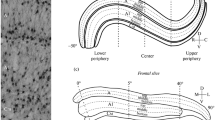
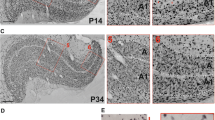
Quantitative changes in cell number during development of the dorsal lateral geniculate nucleus were determined using semithin serial sections of tissue obtained from 28 rats on postnatal day 0, 5, 8, 10, 20, 30, 90 or 165. Our results show three phases of postnatal development in the rat dorsal lateral geniculate nucleus: phase 1 from birth until eye opening, which occurs around the 12th day in these litters; phase 2 from eye opening through stabilization of neuron number on the 30th postnatal day, and phase 3 from that event until adulthood. During the first period increases in neuron number and in glial cell number are found accompanying a nearly seven-fold increase in dorsal lateral geniculate nucleus volume. Phase 2 includes a high incidence of neuronal cell death and a continuous increase in the number of glial cells. The third phase is characterized by a stabilization in the number of neurons, although the glial cell number continues to increase. Neuronal density decreases exponentially throughout the postnatal life of the rat, while the density of glial cells remains relatively stable over the period of study. The postnatal phenomenon of an initial increase in neuron number followed by a period of neuron death may be related to modulating and plastic functions which occur in the rat dorsal lateral geniculate nucleus before a stable neuronal population is achieved on the 30th postnatal day.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Altman J (1966) Autoradiographic and histological studies of the postnatal neurogenesis. II. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in infant rats, with special reference to postnatal neurogenesis in some brain regions J Comp Neurol 128:431–474
Altman J, Das GD (1965a) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335
Altman J, Das GD (1965b) Postnatal origin of microneurones in the rat brain. Nature 207:953–956
Altman J, Das GD (1967) Postnatal neurogenesis in the Guineapig. Nature 214:1098–1101
Angevine JB Jr (1965) Time of neuron origin in the hippocampal region, an autoradiographic study in the mouse. Exp Neurol 13 [Suppl 2]:1–70
Bayer SA (1983)3H-Thymidine-radiographic Studies of Neurogenesis in the Rat Olfactory Bulb. Exp Brain Res 50:329–340
Bayer SA, Yackel JW, Puri PS (1982) Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216:890–892
Biesold D, Brückner G, Mares V (1976) An autoradiographic study of gliogenesis in the rat lateral geniculate nucleus (LGN). Brain Res 104:295–301
Blue ME, Parnavelas JG (1983a) The formation and maturation of synapses in the visual cortex of the rat. I. Qualitative analysis. J Neurocytol 12:599–616
Blue ME, Parnavelas JG (1983b) The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol 12:697–712
Brückner G, Mares V, Biesold D (1976) Neurogenesis in the visual system of the rat. An autoradiographic investigation. J Comp Neurol 166:245–256
Cunningham TJ (1982) Naturally occurring neuron death and its regulation by developing neuronal pathways. Int Rev Cytol 74:163–186
Dalton MM, Hommes OR (1968) Correlation of glial proliferation with age in the mouse brain. J Comp Neurol 134: 397–400
Dreher B, Potts RA, Bennett MR (1983) Evidence that early postnatal reduction in the number of rat retinal ganglion cells is due to a wave of ganglion cell death. Neurosci Lett 36:255–260
Fujita S (1967) Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol 32:277–287
Giordano DL, Cunningham TJ (1978) Naturally occurring neuron death in the superior colliculus of the postnatal rat. Anat Rec 190:402
Giordano DL, Murray M, Cunningham TJ (1980) Naturally occurring neuron death in the optic layers of the superior colliculus of the postnatal rat. J Neurocytol 9:603–614
Heumann D, Rabinowicz Th (1980) Postnatal development of the dorsal lateral geniculate nucleus in the normal and enucleated albino mouse. Exp Brain Res 38:75–85
Heumann D, Rabinowicz Th (1982) Postnatal development of the visual cortex of the mouse after enucleation at birth. Exp Brain Res 46:99–106
Hinds JW (1968a) Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol 134:287–304
Hinds JW (1968b) Autoradiographic study of histogenesis in the mouse olfactory bulb. II. Cell proliferation and migration. J Comp Neurol 134:305–322
Kaplan MS (1981) Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol 195:323–338
Kaplan MS, Bell DH (1983) Neuronal proliferation in the 9-month-old rodent. Radioautographic study of granule cells in the hippocampus. Exp Brain Res 52:1–5
Kaplan MS, Hinds JW (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092–1094
Karlsson V (1967) Observations on the postnatal development of neuronal structures in the lateral geniculate nucleus of the rat by electron microscopy. J Ultrastruct Res 17:158–175
Ling EA, Leblond CP (1973) Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol 149:73–82
Ling EA, Paterson JA, Privat A, Mori S, Leblond CP (1973) Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol 149:43–72
Lund RD, Lund JS (1972) Development of synaptic patterns in the superior colliculus of the rat. Brain Res 42:1–20
Lund RD, Mustari MJ (1977) Development of the geniculocortical pathway in rats. J Comp Neurol 173:289–306
Matthews MA, Narayanan CH, Narayanan Y, Siegenthaler-Matthews DJ (1982) Inhibition of axoplasmic transport in the developing visual system of the rat. III. Electron microscopy and Golgi studies of retino-fugal synapses and post-synaptic neurons in the dorsal lateral geniculate nucleus. Neuroscience 7:405–422
Miale IL, Sidman RL (1961) An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol 4:277–296
Morest DK (1970) The pattern of neurogenesis in the retina of the rat. Z Anat Entwickl-Gesch 131:45–67
Oppenheim RW (1981) Neuronal cell death and some related regressive phenomena during neurogenesis: a selective historical review and progress report. In: Cowan WM (ed) Studies in development neurobiology: assays in honor Viktor Hamburger. Oxford University Press, New York, pp 74–133
Parnavelas JG, Mounty EJ, Bradford R, Lieberman AR (1977) The postnatal development of neurons in the dorsal lateral geniculate nucleus of the rat: a golgi study. J Comp Neurol 171:481–500
Perry VH, Henderson Z, Linden R (1983) Postnatal changes in retinal ganglion cell and optic axon population in the pigmented rat. J Comp Neurol 219:356–368
Potts RA, Dreher B, Bennett MR (1982) The loss of ganglion cells in the developing retina of the rat. Dev Brain Res 3:481–486
Satorre J, Cano J, Reinoso-Suárez F (1985a) Stability of the neuronal population of the dorsal lateral geniculate nucleus (LGNd) of aged rats. Brain Res 339:375–377
Satorre J, Cano J, Reinoso-Suárez (1985b) Sinaptogénesis del núcleo geniculado lateral dorsal (NGLd) de la rata. Arch Soc Esp Oftal (in press)
Sturrock RR (1976) Light microscopic identification of immature glial cells in semithin sections of the developing mouse corpus callosum. J Anat 122:521–537
Sugita S, Otani K (1983) Quantitative analysis of the lateral geniculate nucleus in the mutant microphthalmic rat. Exp Neurol 82:413–423
Taber Pierce E (1973) Time of origin of neurons in the brain stem of the mouse. Prog Brain Res 40:53–65
Weidman TA, Kuwabara T (1968) Postnatal development of the rat retina. An electron microscopic study. Arch Ophthalmol 79:470–484
Winick M (1974) Cellular growth during normal and abnormal development of the brain. In: Himwick W (ed) Biochemistry of the developing brain, vol 2. Dekker, New York, pp 199–226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satorre, J., Cano, J. & Reinoso-Suárez, F. Quantitative cellular changes during postnatal development of the rat dorsal lateral geniculate nucleus. Anat Embryol 174, 321–327 (1986). https://doi.org/10.1007/BF00698782
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00698782