Abstract
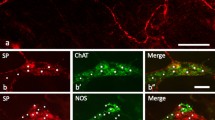
The immunoreactivity of substance P(SP) in the monkey trigeminal ganglion was examined and the distribution of immunoreactive cells determined. The monkey trigeminal ganglion is composed of clusters of sensory cells arranged in cords parallel to the long axis of the nerve fibres. The cells have prominent nuclei and are surrounded by satellite cells. Abundant organelles are randomly distributed throughout the cytoplasm. A striking feature of the ganglion was the presence of some axon-like prolifes containing mainly dense-cored vesicles and some agranular vesicles. Between 16 and 32% of the ganglion cells displayed SP-immunoreactivity. Most of the SP-IR cells were unipolar, small to medium-sized ganglion cells and they had no specific pattern of distribution. The staining of the SP-IR cells varied considerably, ranging from weak or moderate to heavy staining, although the majority of them were moderately stained. Immuno-electron microscopy showed that the SP-IR products were distributed throughout the soma of ganglion cells and not associated with any particular organelles or inclusions. The reaction products were also found in both myelinated and unmyelinated fibres between the ganglion cells. Another remarkable feature of the trigeminal ganglion was the occurrence of some SP-IR nerve fibres forming a rich “glomerular” network of pericellular arborizations around some of the SP-negative cells. Ultrastructural study showed the presence of some SP-IR nerve terminals in close approximation to some SP-negative cells, but there were no synaptic contacts. The relative frequency of the SP-IR pericellular arborizations paralleled the frequency of all the SP-IR cells. The results may imply that the pericellular arborizations function as a medium of communication between SP-positive and SP-negative sensory cells within the ganglion. It was suggested that the fibres forming the pericellular arborizations may originate from the intrinsic ganglion cells that are SP-positive.
Similar content being viewed by others
References
Anderson JM (1982) Histometry. In: Bancroft JD, Stones A (eds) Theory and practise of histological techniques, 2nd edn. Churchill Livingstone, London Melbourne New York, pp 548–563
Ayer-Le Lievre CS, Sciger A (1984) Development of substance P-immunoreactive neurons in cranial sensory ganglia of the rat. Int J Dev Neurosci 2:451–463
Cajal SR (1909) Histologie du Système Nerveux, 1st edn. Maloine, Paris, pp 442–452
Cuello AC, Del Fiacco M, Paxinos G (1978) The central and peripheral ends of the substance P-containing sensory neurones in the rat trigeminal system. Brain Res 152:499–509
De Castro F (1965) Sensory ganglia of the cranial and spinal nerves. Normal and pathological. In: Penfield W (ed) Cytology and cellular pathology of the nervous system, vol 1. Hafner, New York, pp 93–143
Del Fiacco M, Cuello AC (1980) Substance P- and enkephalin-containing neurones in the rat trigeminal system. Neuroscience 5:803–815
Del Fiacco M, Quartu M, Floris A, Diaz G (1990) Substance P-like immunoreactivity in the human trigeminal ganglion. Neurosci Lett 110:16–21
Del Fiacco M, Quartu M, Floris A, Diaz G (1991) Substance P-containing and calcitonin gene-related peptide-containing neurons in the human trigeminal ganglion. In: Leeman SE, Krause JE, Lembeck F (eds) Substance P and related peptides (Cellular and molecular physiology). Ann Acad Sci New York, vol 632, pp 382–384
Dogiel AS (1896) Der Bau der Spinalganglien bei den sangetieren. Anat Anz 12:140–152
Ehrlich P (1886) Ueber Methylenblaureaction der labenden Nervensubstanz. Dtsch Med Wochenschr 12:49–52
Gaik GC, Farbman AI (1973) The chicken trigeminal ganglion: I. An anatomical analysis of the neuron types in the adult. J Morphol 141:43–56
Hökfelt T, Kellerth JO, Nilsson G, Pernow B (1975) Substance P: localization in the central nervous system and in some primary sensory neurons. Science 190:889–890
Jacobs JM, Carmichael N, Cavanagh JB (1975) Ultrastructural changes in the dorsal root and trigeminal ganglia of rats poisoned with methyl mercury. Neuropathol Appl Neurobiol 1:1–19
Katz DM, Karten HJ (1980) Substance P in the vagal sensory ganglia. Localization in cell bodies and pericellular arborizations. J Comp Neurol 193:549–564
Kayahara T (1986) Synaptic connections between motor neurons and dorsal root ganglion cells in the cat. Brain Res 376:299–309
Kayahara T, Takimoto T, Sakashita S (1981) Synaptic junctions in the cat spinal ganglion. Brain Res 216:277–290
Kayahara T, Sakashita S, Takimota T (1984) Evidence of spinal origin of neurons synapsing with dorsal root ganglion cells of the cat. Brain Res 293:225–230
Kerr FWL (1967) Correlated light and electron microscopic observations on the normal trigeminal ganglion and sensory root in man. J Neurosurg 26:132–137
Kuwayama Y, Stone RA (1986) Neuropeptide immunoreactivity of pericellular baskets in the guinea pig trigeminal ganglion. Neurosci Lett 64:169–172
Kuwayama Y, Stone RA, Terenghi G, Polak JM (1985) Substance P-, calcitonin gene-related peptide and cholecystokinin/gastrin-like immunoreactive trigeminal ganglion cells supply the eye: quantitative evaluation using immunohistochemistry and retrograde tracing. Soc Neurosci Abst 11:16
Lehtosalo JI, Uusitalo H, Sijernschantz J, Palkama A (1984) Substance P-like immunoreactivity in the trigeminal ganglion. A flourescence, light and electron microscope study. Histochem J 80:421–427
Lieberman AR (1976) Sensory ganglian. In: Landen DN (ed) The peripheral nerve. Chapman and Hall, London, pp 188–278
Ling EA, Wong WC (1988) An electron microscopic study of the nodose (inferior vagal) ganglion cells in the monkey. J Neurocytol 17:845–857
Ling EA, Yick TY, Ng GL, Wong WC (1992) Immunocytochemical localisation of substance P in vagal ganglion cells and pericellular aborisations in the monkey. J Anat 181:61–71
Lundberg JM, Hökfelt T, Nilsson G, Terenius L, Rehfeld J, Elde R, Said S (1978) Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand 104:499–501
Lundberg JM, Hökfelt T, Nilsson G, Terenius L, Rehfeld J, Elde R, Said S (1984) Sensory substance P nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res 235:251–261
Miller RV, Kruger S, Coates PW, Orkand PM (1970) Formation of synaptic contents on dissociated chick embryo sensory ganglion cells in vitro. Brain Res 24:356–358
Moses HL (1967) Comparative fine structure of the trigeminal ganglia, including human autopsy studies. J Neurosurg 26:112–126
Pineda A, Maxwell DS, Kruger L (1967) The fine structure of neurons and satellite cells in the trigeminal ganglion of cat and monkey. Am J Anat 121:461–488
Sugiura Y, Kitoh J, Sakai H (1983) A comparative ultrastructural study of the trigeminal ganglion in the rat and chicken. J Morphol 175:101–113
Terenghi G, Gibson SJ, McGregor GP, Ghatei MA, Mulderry PK, Bloom SR, Polak JM (1985) Substance P and calcitonin generelated peptide (CGRP) immunoreactivity are co-localised in primary sensory neurons. In: Jordan CC, Oehme P (eds) Substance P: metabolism and biological actions. Taylor & Francis, London Philadelphia, pp 208
Tervo K, Tervo T, Eranko L, Eranko O, Cuello AC (1981) Immunoreactivity for substance P in the Gasserian ganglion, opthalmic nerve and anterior segment of the rabbit eye. Histochem J 13:435–443
Truex RC (1940) Morphological alterations in the gasserian ganglion cells and their association with senescent man. Am J Pathol 16:255–268
Von Euler US, Gaddum GH (1931) An undentified depressor substance in certain tissue extracts. J Physiol (Lond) 72:74–87
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ng, Y.K., Wong, W.C. & Ling, E.A. A qualitative and quantitative study of substance P immuno-cytochemistry of the trigeminal ganglion in the monkey. Anat Embryol 188, 53–61 (1993). https://doi.org/10.1007/BF00191451
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191451