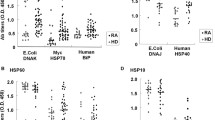
Abstract
Cellular immune responses to microbial antigens have been implicated in the pathogenesis of some forms of arthritis including reactive arthritis, Reiter's syndrome, ankylosing spondylitis and rheumatoid arthritis. We investigated the proliferative T cell responses of paired peripheral blood (PB) and synovial fluid (SF) mononuclear cells (MC) to so-called arthritogenic bacteria (Yersinia entero-colitica and Salmonella typhimurium), to control antigens, such as Candida albicans, mumps virus and purified protein derivative, to the recombinant mycobacterial 65-kDa heat-shock protein (hsp 65) and the mitogen phytohemagglutinin (PHA) in 16 patients with different inflammatory rheumatic diseases. The [3H]thymidine uptake of unstimulated cells (medium control) as well as the proliferative response to the different antigens tested was markedly increased in SFMC irrespective of the underlying rheumatic disease. In contrast, mitogenic stimulation was decreased in SFMC. The proliferative response to the hsp 65 correlated significantly with the responses to Yersinia, Salmonella and Candida. These results may reflect an enhanced function of SF antigen-presenting cells, different functional properties and subset distributions of PB and SF T cells with a preferetial accumulation of helper-inducer/memory T cells or a maintenance of an ongoing immune response by T cells cross-recognizing self epitopes such as epitopes located on the hsp 65. Thus, care should be taken in the interpretation of SF T cell responses to microbial antigens as diagnostic tools in arthritis.
Similar content being viewed by others

References
Abrahamsen TG, Froland SS, Natvig JB (1978) In vitro mitogen stimulation of synovial fluid lymphocytes from rheumatoid arthritis and juvenile rheumatoid arthritis patients: dissociation between the response to antigens and polyclonal mitogens. Scand J Immunol 7:81–90
Ausiello CM, Spangnoli GC, Boccanera M, Casalinuovo I, Malavasi F, Casciani CU, Cassone A (1986) Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol 22:195–202
Burmester GR, Kalden JR, Peter HH, Schedel I, Beck P, Wittenborg A (1978) Immunologic and functional characteristics of peripheral blood and synovial fluid lymphocytes from patients with rheumatoid arthritis. Scand J Immunol 7:405–417
Falk LA, Wahl LM, Vogel SN (1988) Analysis of Ia antigen expression in macrophages derived from bone marrow cells cultured in granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. J Immunol 140:2652–2660
Firestein GS, Zvaifler NJ (1987) Peripheral blood and synovial monocyte activation in inflammatory arthritis. I. A cytofluorographic study of the differentiation antigens and class II antigens and their regulation by gamma-interferon. Arthritis Rheum 30:857–863
Firestein GS, Zvaifler NJ (1987) Peripheral blood and synovial monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage-activating factor. Arthritis Rheum 30:864–871
Ford DK, da Roza DM, Ward RH (1984) Arthritis confined to knee joints. Synovial lymphocyte responses to microbial antigens correlate with distribution of HLA. Arthritis Rheum 27:1157–1164
Ford DK, da Roza DM, Schulzer M (1985) Lymphocytes from the site of disease but not blood lymphocytes indicate the cause of arthritis. Ann Rheum Dis 44:701–710
Førre O, Dobloug JH, Natvig JB (1982) Augmented numbers of HLA-DR-positive T lymphocytes in the synovial fluid and synovial tissue of patients with rheumatoid arthritis and juvenile rheumatoid arthritis. In vivo-activated T lymphocytes are potent stimulators in the mixed lymphocyte reaction. Scand J Immunol 15:227–231
Gaston JSH, Life PF, Granfors K, Merilahti-Palo R, Bailey L, Consalvey S, Toivanen A, Bacon PA (1989) Synovial T lymphocyte recognition of organisms which trigger reactive arthritis. Clin Exp Immunol 76:348–352
Gaston JSH, Life PF, Bailey LC, Bacon PA (1989) In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol 143:2494–2500
Gaston JSH, Life PF, Jenner PJ, Colston MJ, Bacon PA (1990) Recognition of a mycobacteria-specific epitope in the 65-kDa heat-shock protein by synovial fluid-derived T cell clones. J Exp Med 171:831–841
Goto M, Miyamoto T, Nishioka K (1987) Two dimensional flow cytometric analysis of activation antigens expressed on the synovial fluid T cells in rheumatoid arthritis. J Rheumatol 14:230–233
Granfors K, Jalkanen S, von Essen R, Lahesmaa-Rantala R, Isomäki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomäki H, Toivanen A (1989) Yersinia antigens in synovial fluid cells from patients with reactive arthritis. N Engl J Med 320:216–221
Granfors K, Jalkanen S, Lindberg AA, von Essen R, Toivanen A (1990) Salmonella lipopolysaccharide in synovial fluid cells from patients with reactive arthritis. Clin Rheumatol [A] 9:136
Hermann E, Fleischer B, Mayet W-J, Poralla T, Meyer zum Büschenfelde K-H (1989) Response of synovial fluid T cell clones to Yersinia enterocolitica antigens in patients with reactive Yersinia arthritis. Clin Exp Immunol 75:365–370
Hermann E, Mayet W-J, Probst P, Fleischer B, Poralla T, Meyer zum Büschenfelde K-H (1990) Heterogeneity and HLA-DR restriction of Yersinia-reactive synovial fluid T cell clones in Yersinia arthritis. Clin Rheumatol [A] 9:125
Hermann E, Mayet W-J, Poralla T, Meyer zum Büschenfelde K-H, Fleischer B 1990 Salmonella reactive synovial fluid T cell clones in a patient with postinfectious Salmonella arthritis. Scand J Rheumatol (in press)
Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS (1989) Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65kilodalton mycobacterial antigen. Mol Cell Biol 9:2279–2283
Keat A, Dixey J, Sonnex C, Thomas B, Osborn M, Taylor-Robinson D (1987) Chlamydia trachomatis and reactive arthritis: the missing link. Lancet I:72–74
Lasky HP, Bauer K, Pope RM (1988) Increased helper-inducer and decreased suppressor-inducer phenotypes in the rheumatoid joint. Arthritis Rheum 31:52–59
Life PF, Viner NJ, Bacon PA, Gaston JSH (1990) Synovial fluid antigen-presenting cells unmask peripheral blood T cell responses to bacterial antigens in inflammatory arthritis. Clin Exp Immunol 79:189–194
Pitzalis C, Kingsley G, Lanchbury JSS, Murphy J, Panayi GS (1987) Expression of HLA-DR, DQ and DP antigens and interleukin-2 receptor on synovial fluid T lymphocyte subsets in rheumatoid arthritis: evidence of “frustrated” activation. J Rheumatol 14:662–666
Pitzalis C, Kingsley G, Haskard D, Panayi G (1988) The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol 18:1397–1404
Potocnik AJ, Kinne R, Menninger H, Zacher J, Emmrich F, Kroczek RA (1990) Expression of activation antigens on T cells in rheumatoid arthritis patients. Scand J Immunol 31:213–224
Res PC, Schaar CG, Breedveld FC, van Eden W, van Embden JDA, Cohen IR, De Vries RRP (1988) Synovial fluid T cell reactivity against the 65-kDa heat-shock protein of mycobacteria in early chronic arthritis. Lancet II:478–488
Silver R, Redelman D, Zvaifler N (1983) Studies of rheumatoid synovial fluid lymphocytes. II. A comparison of their behaviour with blood mononuclear cells in the autologous mixed lymphocyte reaction and responses to TCGF. Clin Immunol Immunopathol 27:15–27
Thole JER, Keulen WJ, Kolk AHJ, Groothius DG, Berwald LG, Tiesjema RH, van Embden JDA (1987) Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in Escherichia coli K-12. Infect Immun 55:1466–1475
Van Eden W, Thole JER, van der Zee R, Noordzij A, van Embden JDA, Hensen EJ, Cohen IR (1988) Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331:171–173
Van Eden W, Hogervorst EJM, van der Zee R, van Embden JDA, Hensen EJ, Cohen IR (1989) The mycobacterial 65-kDa heat-shock protein and autoimmune arthritis. Rheumatol Int 9:187–191
Winrow VR, McLean L, Morris CJ, Blake DR (1990) The heat-shock protein response and its role in inflammatory disease. Ann Rheum Dis 49:128–132
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hermann, E., Mayet, WJ., Lohse, A.W. et al. Proliferative response of synovial fluid and peripheral blood mononuclear cells to arthritogenic and non-arthritogenic microbial antigens and to the 65-kDa mycobacterial heat-shock protein. Med Microbiol Immunol 179, 215–224 (1990). https://doi.org/10.1007/BF00195252
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00195252