Summary
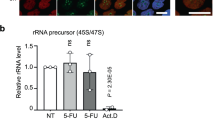
To determine the relative importance of the DNA and RNA effects in the cytotoxicity of 5-fluorouracil, we determined the effects of brief exposure to 5-fluorouridine and 5-fluoro-2′-deoxyuridine, compared with 5-fluorouracil, on the subsequent growth of murine lymphoma L5178Y cells during prolonged incubations following the removal of the drug from the culture medium. 5-Fluoro-2′-deoxyuridine markedly inhibited cell proliferation by continuous exposure. However, its inhibitory effect by exposure even at 1,000 μM for 1 h easily disappeared within 1 week after removal of the drug from the culture medium. This result indicates that the cytotoxic effect of 5-fluoro-2′-deoxyuridine is reversible on cell proliferation.
On the other hand, the exposure to 5-fluorouridine, event at the low concentration of 1.5 μM for 1 h, caused a substantial and irreversible inhibition on cell proliferation.
The cytotoxic effect of 5-fluorouracil was also an irreversible action similar to that of 5-fluorouridine.
Similar content being viewed by others
Abbreviations
- FU:
-
5-fluorouracil
- FUR:
-
5-fluorouridine
- FUdR:
-
5-fluoro-2′-deoxyuridine
- IC99 :
-
99% inhibition concentration
References
Bujard H, Heidelberger C (1966) Fluorinated pyrimidines. XXVII. Attempts to determine transcription errors during the formation of fluorouracil-containing messenger ribonucleic acid. Biochemistry 5:3339–3344
Cohen SS (1971) On the nature of thymineless death. Ann NY Acad Sci 186:292–301
Doolittle CH, Mandel HG, Hahn GA (1973) A dissection of the inhibitory effects of 5-fluorouracil in Bacillus Cereus. Chemotherapy 19:22–37
Danneberg PB, Montag BJ, Heidelberger C (1958) Studies on fluorinated pyrimidines IV. Effects on nucleic acid metabolism in vivo. Cancer Res 18:329–334
Hartmann K-U, Heidelberger C (1961) Studies on fluorinated pyrimidines. XIII. Inhibition of thymidylate synthetase. J. Biol Chem 236:3006–3013
Heidelberger C (1965) Fluorinated pyrimidines. Progr Nucleic Acid Res Mol Biol 4:1–50
Kanzawa F, Hoshi A, Kuretani K (1979) Antitumor activity of alkylesters of 1-β-D-ribofuranosyl-5-fluorouracil 5′-phosphate against murine lymphoma L5178Y resistant to 1-β-D-ribofuranosyl-5-fluorouracil. Bull Cancer 66:497–501
Kessel D, Bruns R, Hall TC (1971) Determinants of responsiveness to 5-fluorouridine in transplantable murine leukemias. Mol Pharmacol 7:117–121
Maley F (1977) Pyrimidine antagonists. In: Becker F (ed) Cancer: A comprehensive treatise. Chemotherapy, Vol 5. Plenum Press, New York, pp 327–361
Mandel HG (1969) The incorporation of 5-fluorouracil into RNA and its molecular consequences. In: Hahn FE (ed) Progress in Molecular and Subcellular Biology, Vol 1. Springer, Berlin Heidelberg New York, pp 82–135
Rich MA, Bolaffi JL, Knoll JE, Cheong L, Eidinoff ML (1958) Growth inhibition of human tumor cell strain by 5-fluorouracil, 5-fluorouridine and 5-fluoro-2′-deoxyuridine reversal studies. Cancer Res 18:730–735
Rueckert RR, Mueller GC (1960) Studies on unbalanced growth in tissue culture. I. Induction and consequences of thymidine deficiency. Cancer Res 20:1584–1591
Santi DS, McHenry CS, Sommer H (1974) Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry 13:471–481
Tseng W-C, Medina D, Randerath K (1978) Specific inhibition of transfer RNA methylation and modification in tissues of mice treated with 5-fluorouracil. Cancer Res 38:1250–1257
Wilkinson DS, Pitot HC (1973) Inhibition of ribosomal ribonucleic acid maturation in Novikoff hepatoma cells by 5-fluorouracil and 5-fluorouridine. J Biol Chem 248:63–68
Wilkinson DS, Tlsty TD, Hanas RJ (1975) The inhibition of ribosomal RNA synthesis and maturation in Novikoff hepatoma cells by 5-fluorouridine. Cancer Res 35:3014–3020
Wilkinson DS, Crumley J (1976) The mechanism of 5-fluorouridine toxicity in Novikoff hepatoma cells. Cancer Res 36:4032–4038
Zimmerman M, Seidenberg J (1964) Deoxyribosyl transfer. I. Thymidine phosphorylase and nucleoside deoxyribosyltransferase in normal and malignant tissues. J Biol Chem 239:2618–2621
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanzawa, F., Hoshi, A. & Kuretani, K. Mechanism of cytotoxicity of 5-fluorouracil: Distinction between the irreversible cytotoxic effect of 5-fluorouridine and the reversible cytotoxic effect of 5-fluoro-2′-deoxyuridine on murine lymphoma L5178Y cells in culture. J Cancer Res Clin Oncol 98, 85–90 (1980). https://doi.org/10.1007/BF00413180
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00413180