Summary
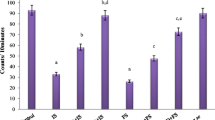
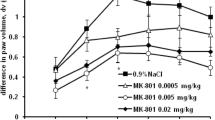
The effect of immobilisation stress on acute pedal inflammation induced by carrageenin, and the mechanism of stress-induced anti-inflammatory effect, were investigated in male Wistar strain albino rats. Carrageenin-induced pedal inflammation oedema was attenuated by immobilisation stress in a time-dependent manner, when the rats were restrained for 30 min, 1 h, and 2 h immediately after the induction of the inflammation. Pentobarbitone exhibited significant anti-inflammatory effect of its own in an anaesthetic dose and also inhibited stress (1 h)-induced attenuation of the inflammation. Likewise, lignocaine, injected behind the knee joint of the inflamed limb, attenuated the inflammation and also inhibited the stressinduced anti-inflammatory effect. These findings indicate the importance of the central nervous system (CNS) and the afferent/efferent neural pathways from and to the inflammatory site, in inflammation and in stress-induced anti-inflammatory effect.
Earlier studies from this laboratory have shown that the central noradrenergic, histaminergic, serotonergic and GABA-ergic neurotransmitter systems have a modulatory anti-inflammatory effect on carrageenin-induced pedal oedema. Since all these neurotransmitter systems have been reported to be activated by stress, their role was assessed in the inflammation-attenuation effect of immobilisation stress. The present studies indicate that, of these neurotransmitters, only the central noradrenergic system is involved in the anti-oedema effect of stress. Endogenous opioid peptides may also be involved in the stress-inflammation interaction, since naloxone inhibited the stress effect.
Bilateral adrenalectomy and peripheral chemical sympathectomy, induced by i.p. administration of 6-hydroxydopamine, augmented carrageenin oedema and antagonised the stress-induced anti-inflammatory effect. However, metyrapone, an inhibitor of endogenous corticoid synthesis, failed to inhibit the stress effect. These findings indicate that the sympatho-medullary system, which is known to be activated during stress, is responsible for the observed anti-inflammatory effect of immobilisation stress, rather than augmented release of adrenal corticoids.
It is suggested that the observed inflammation reducing effect of immobilisation stress is a consequence of increased central noradrenergic and peripheral sympatho-medullary activity.
Similar content being viewed by others
References
Amar A (1981) Temperature changes during immobilisation stress in rats. PhD thesis, Banaras Hindu University
Amar A, Mandal S, Sanyal AK (1982) Effect of brain monoamines on the secretion of adrenocorticotrophic hormone. Acta Endocrinol 101:180–186
Anisman H, Kokkinidis L, Sklar LS (1985) Neurochemical consequences of stress. Contributions of adaptive processes. In: Burchfield SR (ed) Stress psychological and physiological interactions. Hemisphere Publishing Corp, Washington, pp 67–98
Bhattacharya SK (1982) Stress by restraining elevates brain prostaglandins in the rat. Neurosci Lett 33:165–168
Bhattacharya SK, Bhattacharya D (1982) Effect of restraint stress on rat brain serotonin. J Biosci 4:269–274
Bhattacharya SK, Das N (1984) Effect of central prostaglandins on carrageenin-induced pedal inflammation in rats. J Pharm Pharmacol 36:766–767
Bhattacharya SK, Das N (1985) Anti-inflammatory effect of intraventricularly administered histamine in rats. Agents Actions 17:150–152
Bhattacharya SK, Das N (1985) Central serotonergic modulation of carrageenin-induced pedal inflammation in rats. Pharmacol Res 6:315–318
Bhattacharya SK, Das N (1986) Central catecholaminergic modulation of carrageenininduced pedal oedema in rats. Res Exp Med (Berl) 186:365–374
Bhattacharya SK, Sarkar MK (1986) Effect of some centrally administered putative amino acid neurotransmitters on carrageenin-induced paw oedema in rats. J Pharm Pharmacol 38:144–146
Brown JH, Kissel JW, Lish PM (1968) Studies on acute inflammatory response. I. Involvement of central nervous system in certain models of inflammation. J Pharmacol Exp Ther 160:231–242
Brown RA, West GB (1965) Sympathomimetic amines and vascular permeability. J Pharm Pharmacol 17:119–120
Das N (1983) Central modulation of peripheral inflammation in rats. PhD thesis, Banaras Hindu University
Das N, Bhattacharya SK (1985) Central cholinergic modulation of carrageenin-induced pedal inflammation in rats. Pharmacol Res 3:137–139
DiRosa M, Giroud JP, Willoughby DA (1971) Studies on the mediators of the acute inflammatory response induced in rats and different sites by carrageenin and turpentine. J Pathol 104:15–29
Dunn AJ, Kramarcy NR (1984) Neurochemical responses in stress: relationships between the hypothalamo-pituitary-adrenal and catecholamine system. In: Iversen LL, Iversen SD, Snyder SH (eds) Handbook of psychopharmacology, vol 18. Plenum Press, New York, pp 455–479
Dunn AJ, Kramarcy NR, Villiger JW (1981) ACTH-induced grooming involves high affinity opiate receptors. Behav Neural Biol 31:105–109
Feldberg W, Lotti VJ (1967) Temperature response to monoamines and an inhibitor of MAO injected into the cerebral ventricles of rats. Br J Pharmacol Chemother 31:152–161
Foreman JC, Jordan CC (1984) Neurogenic inflammation. TIPS 5:116–119
Fregley MJ (1953) Minimal exposures needed to acclimatize rats to cold. Am J Physiol 173:393–402
French ED, Bloom RE, Rivier C, Guillemin R, Rossier J (1978) Morphine or stressinduced increase of plasma β-endorphin and prolactin are prevented by dexamethasone treatment. Soc Neurosci [Abstr] 4:1285
Glenn M, Gray J (1965) Adjuvant induced polyarthritis in rats: biologic and historical background. Am J Vet Res 26:1180–1194
Griswold DE, Alessi S, Webb EF, Walz DT (1982) Inhibition of carrageenin-induced inflammation by urethane anaesthesia in adrenalectomised and sham-operated rats. J Pharmacol Methods 8:161–164
Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F (1977) β-Endorphin and adrenocorticotropin are secreted concomitantly by the pituitary gland. Science 197:1367–1369
Ingle DJ, Griffith JO (1949) The rat in laboratory investigation. (Ferris CJ, Griffith JO, eds). Hafner, New York, p 444
Jezova D, Vigas M, Jurcovicova J (1982) ACTH and corticosterone respone to naloxone and morphine in normal, hypophysectomized and dexamethasone-treated rats. Life Sci 31:307–314
Lombardino JG, Otterness KG, Wiseman EH (1975) Anti-inflammatory agents — correlations of some physical, pharmacological and clinical data. Arzneimittelforsch 25:1629–1635
Mason JW (1971) A re-evaluation of the concept of “non-specificity” in stress theory. J Psychiatr Res 8:323–333
McCarty R, Kopin IJ (1979) Stress-induced alterations in plasma catecholamines and behaviour in rats. Effects of chlorisondamine and bretylium. Behav Neural Biol 27:249–265
Palit G, Gupta MB, Bhargava R, Dixit KS, Bhargava KP (1979) Central histaminoceptor-adrenoceptor inter-relationship in the release of adrenocorticotrophic hormone. Ind J Physiol Pharmacol 23:372–376
Pohorecky LA, Wurtman RJ (1971) Adrenocortical control of epinephrine synthesis. Pharmacol Rev 23:1–35
Selye H (1936) Thymus and adrenals in the response of the organism to injuries and intoxication. Br J Exp Pathol 17:234–248
Sofia RD (1980) The effect of overcrowding stress on the development of adjuvantinduced polyarthritis in the rat. J Pharm Pharmacol 32:874–875
Stone EA, Platt JE (1982) Brain adrenergic receptors and resistance to stress. Brain Res 237:405–414
Temple TE, Liddle GW (1970) Inhibitors of adrenal steroid biosynthesis. Annu Rev Pharmacol Toxicol 10:199–218
Thoenen H, Tranzer JP (1973) The pharmacology of 6-hydroxydopamine. Annu Rev Pharmacol Toxicol 13:169–180
Volovka J, Cho D, Mallya A, Bauman J (1979) Naloxone increases ACTH and cortisol levels in man. N Engl J Med 300:1056–1057
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol 111:544–547
Author information
Authors and Affiliations
Additional information
Supported by a grant-in-aid from the Council of Scientific and Industrial Research, New Delhi
Rights and permissions
About this article
Cite this article
Bhattacharya, S.K., Das, N. & Sarkar, M.K. Inhibition of carrageenin-induced pedal oedema in rats by immobilisation stress. Res. Exp. Med. 187, 303–313 (1987). https://doi.org/10.1007/BF01852056
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01852056