Summary
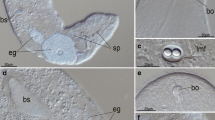
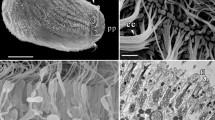
The genus Allanaspides (Crustacea, Syncarida) is characterised by a conspicuous modification of the cephalo-thoracic tergite, the fenestra dorsalis. The ultrastructure of the fenestra dorsalis was examined in both known species of Allanaspides. The organ is separated from surrounding tissue by a clearly demarcated transition zone in which the cuticle thickens and looses its normal laminated structure. In the cells of the fenestra dorsalis, three regions may be distinguished. The apical region has an abundance of long invaginations, often packed closely together. The invaginations possess numerous microtubules plus numerous mitochondria whose membranes are closely associated with the invaginated membranes. Both microtubules and mitochondria are aligned in the same direction as the invaginations. The middle region of the cells contains many intracellular vacuoles in Allanaspides hickmani whilst in Allanaspides helonomus the same region contains extensive extracellular spaces. The basal region of the cells is marked by deep infoldings and an abundance of mitochondria. Microtubules are common and these, together with the mitochondria, are aligned along the direction of the infoldings. Adjacent cells are linked by frequent septate junctions. The fine structure of fenestra dorsalis tissue indicates an active participation in ion and/or water movement. However, in contrast to other crustacean tissues known to be involved in this function, it is postulated that the fenestra dorsalis of Allanaspides provides a two-stage transport system, in which “mitochondrial pumps” are present on both the inner and outer cell surfaces.
Similar content being viewed by others
References
Anderson, E., Harvey, W. R.: Active transport by the Cecropia midgut. II. Fine structure of the midgut epithelium. J. Cell Biol. 31, 107–134 (1966)
Berridge, M. J.: A structural analysis of intestinal absorption. In: Insect Ultrastructure, A. C. Neville ed. Roy. Ent. Soc. Sympos. vol. 5, p. 135–151. Oxford: Backwells 1970
Berridge, M. J., Gupta, B. L.: Fine structural changes in relation to ion and water transport in the rectal papillae of the blowfly. Calliphora. J. Cell Sci 2, 89–112 (1967)
Berridge, M. J., Oschman, J. L.: A structural basis for fluid secretion by Malphigian tubules. Tissue and Cell 1, 247–272 (1969)
Bielawski, J.: Ultrastructure and ion transport in gill epithelium of the crayfish Astacus leptodactylus Esch. Protoplasma (Wien) 73, 177–190 (1971)
Copeland, D. E.: A mitochondrial pump in the cells of the anal papillae of mosquito larvae. J. Cell Biol. 23, 253–263 (1964)
Copeland, D. E.: A study of salt secretion cells in the Brine Shrimp (Artemia salina). Protoplasma (Wien) 63, 363–384 (1967)
Copeland, D. E.: Fine structure of salt and water uptake in the Land Crab Gecarcinus lateralis. Amer. Zool. 8, 417–432 (1968)
Copeland, D. E., Fitzjarrell, A. T.: The salt absorbing cells in the gills of the Blue Crab (Callinectes sapidus Rathbun) with notes on modified mitochondria. Z. Zellforsch. 92, 1–22 (1968)
Diamond, J. M.: Water-solute coupling and ion selectivity in epithelia. Phil. Trans. B 262, 141–151 (1971a)
Diamond, J. M.: Standing-gradient model of fluid transport in epithelia. Fed. Proc. 30, 6–13 (1971b)
Diamond, J. M., Bossert, W. H.: Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J. gen. Physiol. 50, 2061–2083 (1967)
Diamond, J. M., Bossert, W. H.: Functional consequences of ultrastructural geometry in “backwards” fluid transporting epithelia. J. Cell Biol. 37, 694–702 (1968)
Fisher, J. M.: Fine-structural observations on the gill filaments of the freshwater crayfish, Astacus pallipes Lereboullet. Tissue and Cell. 4, 287–299 (1972)
Gilula, N. B., Branton, D., Satir, P.: The septate junction: A structural basis for intercellular coupling. Proc. nat. Acad. Sci. (Wash.) 67, 213–220 (1970)
Gupta, B. L., Berridge, M. J.: Fine structural organization of the rectum in the blowfly, Calliphora enythrocephala (Meig.) with special reference to connective tissue, tracheae and neurosecretory innervation in the rectal papillae. J. Morph. 120, 23–81 (1966)
Hand, A. R., Gobel, S.: The structural organization of the septate and gap junctions of Hydra. J. Cell Biol. 52, 397–408 (1972)
Holdich, D. M., Ratcliffe, N. A.: A light and electron microscope study of the hindgut of the herbivorous Isopod, Dynamene bidentata (Crustacea: Peracarida). Z. Zellforsch. 111, 209–227 (1970)
Hopkins, C. R.: The fine-structural changes observed in the rectal papillae of the mosquito Aedes aegypti, L. and their relation to the epithelial transport of water and inorganic ions. J. roy. micr. Soc. 86, 235–252 (1967)
Kessel, R. G.: The permeability of dragonfly Malphigian tubule cells to protein using horseradish peroxidase as a tracer. J. Cell Biol. 47, 299–303 (1970)
Lake, P. S.: Trialdehyde fixation of crustacean tissues for electron microscopy. Crustaceana 23, 244–246 (1973)
Loewenstein, W. R., Kanno, Y.: Studies on an epithelial (gland) cell junction I. Modification of surface membrane permeability. J. Cell Biol. 22, 565–586 (1964)
Maddrell, S.H.P.: Fluid secretion by the Malphigian tubules of insects. Phil. Trans. B 262, 197–207 (1971)
Noirot, C., Timothée-Noirot, C.: Revêtement de la membrane cytoplasmique et absorption des ions dans les papilles rectales d'un Termite (Insecta, Isoptera). C. R. Acad. Sci. (Paris) 263, 1099–1102 (1966)
Nüske, N., Wichard, W.: Die Analpapillen der Köcherfliegenlarven. I. Feinstruktur und histochemischer Nachweis von Natrium and Chlorid bei Philipotamus montanus Donov. Cytobiologie 4, 480–486 (1971)
Oschman, J. L., Berridge, M. J.: The structural basis of fluid secretion. Fed. Proc. 30, 49–56 (1971)
Oschman, J. L., Wall, B. J.: The structure of the rectal pads of Periplaneta americana L. with regard to fluid transport. J. Morph. 127, 475–510 (1969)
Peachey, L. D., Rasmussen, H.: Structure of the toad's urinary bladder as related to its physiology. J. biophys. biochem. Cytol. 10, 529–553 (1961)
Perrelet, A., Orci, L., Baumann, F.: Evidence for granulolysis in the retinula cells of a stomatopod crustacean, Squilla mantis. J. Cell. Biol. 48, 684–688 (1971)
Ramsay, J. A.: Insect rectum. Phil. Trans. B 262, 251–260 (1971)
Reynolds, E. A.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
Sacktor, B., Shimada, Y.: Degenerative changes in the mitochondria of flight muscle from aging blowflies. J. Cell Biol. 52, 465–477 (1972)
Schmidt-Nielsen, B., Gertz, K. H., Davis, L. E.: Excretion and ultrastructure of the antennal gland of the fiddler crab Uca mordax. J. Morph. 125, 473–496 (1968)
Slautterback, D. B.: Cytoplasmic microtubules. I. Hydra. J. Cell Biol. 18, 367–388 (1963)
Sohal, R. S., Copeland, E.: Ultrastructural variations in the anal papillae of Aedes aegypti (L.) at different environmental salinities. J. Insect. Physiol. 12, 429–439 (1966)
Swain, R., Wilson, I. S., Hickman, J. L., Ong, J. E.: Allanaspides helonomus gen. et sp. nov. (Crustacea: Syncarida) from Tasmania. Rec. Queen Victoria. Mus. 35, 1–13 (1970)
Swain, R., Wilson, I. S., Ong, J. E.: A new species of Allanaspides (Syncarida, Anaspididae) from south-western Tasmania. Crustaceana 21, 196–202 (1971)
Talbot, P., Clark, W. H., Lawrence, A. L.: Light and electron microscopical studies on osmoregulatory tissue in the developing brown shrimp, Penaeus aztecus. Tissue and Cell. 4, 271–286 (1972)
Tyson, G. E.: The fine structure of the maxillary gland of the brine shrimp, Artemia salina: The efferent duct. Z. Zellforsch. 93, 151–163 (1969)
Wichard, W., Komnick, H.: Electron microscopical and histochemical evidence of chloride cells in tracheal gills of mayfly larvae. Cytobiologie 3, 215–228 (1971)
Witkus, E. R., Grillo, R. S., Smith, W. J.: Microtubule bundles in the hindgut epithelium of the woodlouse Oniscus asellus. J. Ultrastruct. Res. 29, 182–190 (1969)
Wood, R. L.: Intercellular attachment in the epithelium of Hydra as revealed by the electron microscope. J. biophys. biochem. Cytol. 6, 343–352 (1959)
Author information
Authors and Affiliations
Additional information
We wish to thank Mr. R. Davies of the Zoology Department, University of Tasmania, for considerable technical assistance, and Mrs. T. Vasos also of the Zoology Department, University of Tasmania, for typing the manuscript. This study was supported by a University of Tasmania Research Grant.
Rights and permissions
About this article
Cite this article
Lake, P.S., Swain, R. & Ong, J.E. The ultrastructure of the fenestra dorsalis of the syncarid crustaceans Allanaspides helonomus and Allanaspides hickmani . Z.Zellforsch 147, 335–351 (1974). https://doi.org/10.1007/BF00307469
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00307469