Summary
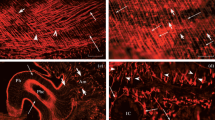
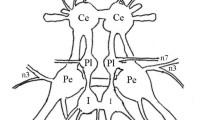
The structure of both the slow- and the fast-adapting abdominal muscle receptor organ of Astacus leptodactylus is described with particular reference to differences between the two systems. The receptors are composed of a thin muscle that extends from the front edge of one segment to the front edge of the following and a sensory cell connected with this muscle. In the zone where the sensory cells enter their respective muscle, muscle fibers are reduced (zone of relative muscle exclusion=ZRME) and partly replaced by connective tissue. The occurrence of dendritic processes of both the slow and the fast neurons is confined to this zone. The following differences between the two receptor types are established:
-
(1)
The fast receptor muscle reveals a smaller sarcomere length than the slow receptor muscle and a higher myosin/actin filament ratio.
-
(2)
Muscle fibers that pass the ZRME are always found at its periphery in the fast system, separated from dendritic processes by layers of connective tissue, while in the slow system muscle fibers frequently are intermingled with the sensory elements.
-
(3)
The ZRME of the slow receptor is 20–30% longer than that of the fast receptor.
-
(4)
The dendritic varicosities of the slow neuron, on an average, contain many more mitochondria than those of the fast neuron.
-
(5)
Dendritic processes (fine twigs as well as varicosities) are juxtaposed to the sarcolemma of the muscle fibers only in the slow system; in the fast system dendrites and muscle are spatially separated by connective tissue.
It is assumed that these differences between the two receptor types arep at least in part responsible for the different thresholds observed in physiological experiments.
Similar content being viewed by others
References
Alexandrowicz, J.S.: Muscle receptor organs in the abdomen of Homarus vulgaris and Palinurus vulgaris. Q.J. Microsc. Sci. 92, 163–199 (1951)
Alexandrowicz, J.S.: Muscle receptor organs in the Paguridae. J. Mar. Biol. Ass. U.K. 31, 563–580 (1952)
Alexandrowicz, J.S.: Notes on the nervous system in the Stomatopoda. IV. Muscle receptor organs. Pubbl. Staz. Zool. Napoli 25, 94–111 (1954)
Alexandrowicz, J.S.: Receptor elements in the muscles of Leander serratus. J. Mar. Biol. Ass. U.K. 35, 129–144 (1956)
Alexandrowicz, J.S.: Further observations on proprioceptors in Crustacea and a hypothesis about their function. J. Mar. Biol. Ass. U.K. 37, 379–396 (1958)
Alexandrowicz, J.S.: Receptor organs in the thoracic and abdominal muscles of Crustacea. Biol. Rev. 42, 288–326 (1967)
Bodian, D., Bergman, R.A.: Muscle receptor organs of crayfish: functional-anatomical correlations. Bull. Johns Hopkins Hosp. 110, 78–106 (1962)
Burkhart, D.: Die Erregungsvorgänge sensibler Ganglienzellen in Abhängigkeit von der Temperatur. Biol. Zbl. 78, 22–62 (1959)
Eyzaguirre, C., Kuffler, S.W.: Further study of soma, dendrite, and axon excitation in single neurons. J. Gen. Physiol. 39, 121–153 (1955)
Fields, H.L.: Proprioceptive control of posture in the crayfish abdomen. J. Exp. Biol. 44, 455–468 (1966)
Fields, H.L.: Reflex role played by efferent control of an invertebrate stretch receptor. J. Neurophysiol. 30, 859–874 (1967)
Fields, H.L.: Crustacean abdominal and thoracic muscle receptor organs. In: Structure and function of proprioceptors in the invertebrates (P.J. Mill, ed.), pp. 65–114. London: Chapman and Hall 1976
Fields, H.L., Kennedy, D.: Functional role of muscle receptor organs in crayfish. Nature 206, 1235–1237 (1965)
Fleissner, G.: Der phasische Streckrezeptor in den abdominalen Muskelrezeptororganen des Sumpfkrebses Astacus leptodactylus. Inaugural-Dissertation, Universität Frankfurt 1972
Florey, E., Florey, E.: Microanatomy of the abdominal Stretch receptors of the crayfish (Astacus fluviatilis L.). J. Gen. Physiol. 39, 69–85 (1955)
Harper, E., Seifter, S., Scharrer, B.: Electron microscopical and biochemical characterization of collagen in blattarian insects. J. Cell Biol. 33, 385–393 (1967)
Karlsson, U., Andersson-Cedergren, E., Ottoson, D.: Cellular organization of the frog muscle spindle as revealed by serial sections for electron microscopy. J. Ultrastruct. Res. 14, 1–35 (1966)
Kennedy, D., Takeda, K.: Reflex control of abdominal flexor muscles in the crayfish. I. The twitch system. J. Exp. Biol. 43, 211–228 (1965a)
Kennedy, D., Takeda, K.: Reflex control of abdominal flexor muscles in the crayfish. II. The tonic system. J. Exp. Biol. 43, 229–246 (1965b)
Kennedy, D., Evoy, W.H., Fields, H.L.: The unit base of some crustacean reflexes. Symp. Soc. Exp. Biol. 20, 75–109 (1966)
Kosaka, K.: Electrophysiological and electron microscopic studies on the neuromuscular junction of the crayfish stretch receptors. Jap. J. Physiol. 19, 160–175 (1969)
Krauhs, J.M., Mirolli, M.: Morphological changes associated with stretch in mechano-receptor. J. Neurocytol. 4, 231–246 (1975)
Kuffler, S.W.: Mechanisms of activation and motor control of stretch receptors in lobster and crayfish. J. Neurophysiol. 17, 558–574 (1954)
Kuffler, S.W., Eyzaguirre, C.: Synaptic inhibition in an isolated nerve cell. J. Gen. Physiol. 39, 155–184 (1955)
Mollenhauer, H.H.: Plastic embedding mixtures for use in electron microscopy. Stain Technol. 39, 111–114 (1964)
Nadol, J.B., de Lorenzo, A.J.: Observations on the abdominal stretch receptor and the fine structure of associated axo-dendritic synapses and neuromuscular junctions in Homarus. J. Comp. Neurol. 132, 419–444 (1968)
Nadol, J.B., de Lorenzo, A.J.: Observations on the organization of the dendritic processes and receptor terminations in the abdominal muscle receptor organ of Homarus. J. Comp. Neurol. 137, 19–58 (1969)
Nakajima, S.: Adaptation in stretch receptor neurons of crayfish. Science 146, 1168–1170 (1964)
Nakajima, S., Onodera, K.: Membrane properties of the stretch receptor neurones of crayfish with particular reference to mechanisms of sensory adaptation. J. Physiol. 200, 161–185 (1969a)
Nakajima, S., Onodera, K.: Adaptation of the generator potential in the crayfish stretch receptors under constant length and constant tension. J. Physiol. 200, 187–204 (1969b)
Nakajima, Y., Tisdale, A.D., Henkart, M.P.: Presynaptic inhibition at inhibitory nerve terminals. A new synapse in the crayfish stretch receptor. Proc. Natl. Acad. Sci. 70, 2462–2466 (1973)
Ottoson, D.: Adaptive properties of crayfish stretch receptor neurons. Rhein.-Westf. Akad. Wiss. 53, 401–414 (1974)
Peterson, R.P., Pepe, F.A.: The fine structure of inhibitory synapses in the crayfish. J. Biophys. Biochem. Cytol. 11, 157–169 (1961)
Pfenninger, K.H.: Synaptic morphology and cytochemistry. Stuttgart-Portland: Fischer 1973
Reynolds, E.S.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
Rhodin, J.A.G.: Histology. A text and atlas. New York-London-Toronto: Oxford University Press 1974
Sabatini, D.D., Bensch, K., Barrnett, R.J.: Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58 (1963)
Smith, D.S.: Insect cells. Their structure and function. Edinburgh: Oliver and Boyd 1968
Thurm, U.: General organization of sensory receptors. Rendiconti S.I.F. 43, 44–68 (1969)
Tisdale, A.D., Nakajima, Y.: Fine structure of synaptic vesicles in two types of nerve terminals in crayfish stretch receptor organs: influence of fixation methods. J. Comp. Neurol. 165, 369–386 (1976)
Uchizono, K.: Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207, 642–643 (1965)
Uchizono, K.: Inhibitory synapses on the stretch neurone of the crayfish. Nature 214, 833–834 (1967)
Uchizono, K.: Morphological background of excitation and inhibition at synapses. J. Electron Microsc. 17, 55–66 (1968)
Whitear, M.: The fine structure of crustacean proprioceptors. II. The thoracico-coxal organ in Carcinus, Pagurus and Astacus. Phil. Trans. R. Soc. B 248, 437–456 (1965)
Wiersma, C.A.G., Furshpan, E., Florey, E.: Physiological and pharmacological observations on muscle receptor organs of the crayfish, Cambarus clarkii Girard. J. Exp. Biol. 30, 136–150 (1953)
Wohlfarth-Bottermann, K.E.: Die Kontrastierung tierischer Zellen im Rahmen ihrer elektronenmikroskopischen Untersuchung an Ultradünnschnitten. Naturwissenschaften 44, 287–288 (1957)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Euteneuer, U., Winter, C. The abdominal muscle receptor organ in Astacus leptodactylus (Crustacea). Cell Tissue Res. 202, 41–61 (1979). https://doi.org/10.1007/BF00239220
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239220