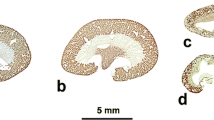
Summary
Following perfusion fixation of the rat kidney with glutaraldehyde the proximal tubule cells display small apical vacuoles, large apical vacuoles, and apical vacuoles in which a part of the limiting membrane is invaginated into the vacuole. These invaginated apical vacuoles occur more frequently in proximal convoluted tubules than in proximal straight tubules. One tubular cell may contain apical vacuoles of different sizes and stages of invagination, ranging from larger vacuoles with a wide lumen and a small area of invaginated membrane to smaller elements with no apparent lumen and a large area of invaginated membrane. Invaginated apical vacuoles lie either singly in the cytoplasm or close to the membranes of other apical vacuoles, but never in contact with the cell membrane or the membranes of lysosomes, endoplasmic reticulum, Golgi apparatus, mitochondria and peroxisomes.
These findings suggest that the invaginated apical vacuoles are not fixation artifacts, but rather develop in living state in cells of the proximal tubule from spherical endocytotic elements.
Similar content being viewed by others
References
Christensen EI (1982) Rapid membrane recycling in renal proximal tubule cells. Eur J Cell Biol 29:43–49
Kaissling B, Kriz W (1979) Structural analysis of the rabbit kidney. Adv Anat Embr Cell Biol 56:1–123
Larsson L (1975) Effects of different fixatives on the ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res 51:140–151
Lewis PR, Knight DP (1977) Staining methods for sectioned material. North Holland, Amsterdam New York Oxford, p 311
Maunsbach AB (1966) The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res 15:242–282
Maunsbach AB (1976) Cellular mechanisms of tubular protein transport. In: Thurau K (ed.) International review of physiology, kidney and urinary tract physiology II. University Park Press, Baltimore, pp 145–167
Neiss WF (1983) The electron density of light and dark lysosomes in the proximal convoluted tubule of the rat kidney. Histochemistry 77:63–77
Neiss WF, Klehn KL (1981) The postnatal development of the rat kidney, with special reference to the chemodifferentiation of the proximal tubule. Histochemistry 73:251–268
Pearse BMF, Bretscher MS (1981) Membrane recycling by coated vesicles. Ann Rev Biochem 50:85–101
Stebbings H, Hunt C (1982) The nature of the clear zone around microtubules. Cell Tissue Res 227:609–617
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft (SFB 105)
Rights and permissions
About this article
Cite this article
Neiss, W.F. Invaginated apical vacuoles in the cells of the proximal convoluted tubule in the rat kidney. Cell Tissue Res. 235, 463–466 (1984). https://doi.org/10.1007/BF00217875
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217875