Summary
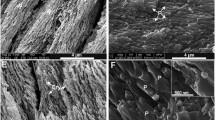
Teeth of three macropod species, M. giganteus, W. bicolor and P. concinna, have been studied using the techniques of light microscopy, scanning- and transmission-electron microscopy and hardness measurement. Light microscope observations showed that the teeth of these species had a translucent enamel region close to the dentine and an outer opaque enamel region at the tooth's surface. These regions were not related to the presence or absence of tubules which are a characteristic feature of marsupial enamel. Hardness tests showed that the opaque enamel was softer than the translucent enamel. Scanning electron microscope observations revealed that there was no correlation between any particular prism packing or orientation and the opaque and translucent enamel regions. Transmission electron microscope observations showed that the translucent enamel region consisted of well defined prisms and well packed, lath-like crystals, whereas the opaque enamel was disrupted by voids (which ranged in size from enlarged micropores to about 2 μm in diameter in extreme cases) between crystals and some randomly oriented, loosely packed crystals. This disruption within the opaque enamel region was more common at prism boundaries but pockets of disrupted enamel were also found within prisms and interprismatic regions. The opacity of the enamel was caused by scattering of light from the voids. The ultrastructure of the opaque enamel region indicated that this region was hypomineralized; hardness tests and polarized light microscope observations were consistent with these results.
Similar content being viewed by others
References
Boyde A, Lester KS (1967) The structure and development of marsupial enamel tubules. Z Zellforsch 82:558–576
Darling AI (1958) Studies of the early lesion of enamel caries its nature mode of spread and points of entry. Br Dent J 105:119–145
Fosse G (1969) The tubules of marsupial enamel investigated by normal light, by polarized light and by contact microradiography. Acta Odontol Scand 27:237–248
Fosse G, Holmbakken N (1971) Fibrils in marsupial enamel tubules. Z Zellforsch 115:341–350
Fosse G, Risnes S, Holmbakken N (1973) Prisms and tubules in multituberculate enamel. Calcif Tissue Res 11:133–150
Gustavsen F (1972) The fine structure of dental enamel and coronal cementum of the kangaroo (Macropus giganteus) Ph D thesis, University of Bergen, Norway
Lester KS (1970) On the nature of “fibrils” and tubules in developing enamel of the opossum, Didelphis marsupialis. J Ultrastruct Res 30:64–77
Moss ML (1969) Evolution of mammalian dental enamel. Am Mus Novit 2360:1–39
Moss ML, Applebaum E (1963) The fibrillar matrix of marsupial enamel. Acta Anat 53:289–297
Orams HJ, Phakey PP, Rachinger WA, Zybert JJ (1974) Visualization of micropore structure in human dental enamel. Nature 252:584–585
Orams HJ, Zybert JJ, Phakey PP, Rachinger WA (1976) Ultrastructural study of human dental enamel using selected area argon-ion-beam thinning. Arch Oral Biol 21:663–675
Osborn JW (1974) The relationship between prisms and tubules in the teeth of Didelphis marsupialis, and the probable origin of the tubules. Arch Oral Biol 19:835–844
Osborn JW, Hillman J (1979) Enamel structures in some therapsids and mesozoic mammals. Calcif Tissue Int 29:47–61
Palamara J, Phakey PP, Rachinger WA, Orams HJ (1980) Electron microscopy of surface enamel of human unerupted and erupted teeth. Arch Oral Biol 25:715–725
Palamara J, Phakey PP, Rachinger WA, Orams HJ (1981a) Electron microscopy of the dentine-enamel junction of kangaroo (Macropus giganteus) teeth using selected-area argon-ion-beam thinning. Cell Tissue Res 221:405–419
Palamara J, Phakey PP, Rachinger WA, Orams HJ (1981b) The ultrastructure of kangaroo enamel (Macropus giganteus): Tubular enamel and its “black spot” defects. Aust J Zool 29:643–652
Phakey PP, Rachinger WA, Orams HJ, Carmichael GC (1974) Preparation of thin sections of selected areas. In: Sanders JV, Goodchild PJ (eds) Electron microscopy Vol. 1. Australian Academy of Science, Canberra, pp 412–413
Powder diffraction file for inorganic materials (1979) (Managing editor McClune WF) JCPDS International Centre for Diffraction Data, Swathmore, Pennsylvania, USA
Risnes S, Fosse G (1974) The origin of marsupial enamel tubules. Acta Anat 87:275–282
Sanson GD (1980) The morphology and occlusion of the molariform cheek teeth in some Macropodinae (Marsupialia: Macropodidae). Aust J Zool 28:341–365
Schmidt WJ, Keil A (1971) Polarizing microscopy of dental tissues. Pergamon Press N.Y. pp 383–388
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palamara, J., Phakey, P.P., Rachinger, W.A. et al. On the nature of the opaque and translucent enamel regions of some macropodinae (Macropus giganteus, Wallabia bicolor and Peradorcas concinna). Cell Tissue Res. 238, 329–337 (1984). https://doi.org/10.1007/BF00217305
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217305