Summary
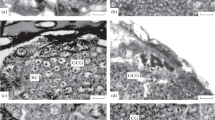
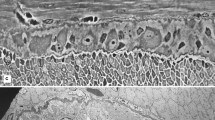
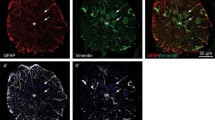
Insect glial cells are capable of division and repair in organ culture after selective damage with the toxin ethidium bromide. The repair is slower and less organised than seen in vivo after similar treatment and is still incomplete after one month. Granule-containing cells, which play an important role in the early stages of repair in vivo, are never seen in cultured connectives. This observation adds further support to the hypothesis that these cells are derived from haemocytes and that their presence is necessary for rapid and orderly repair. The uptake of 3H-thymidine into perineurial glial cells in vitro, both in control and ethidiumtreated connectives, shows that there is a considerable proliferation of cells in this region. Some uptake of thymidine is also seen in subperineurial glia but division alone cannot account for the large increase in the number of glial nuclei found at the early stages of repair in this region. Further, glial cells with diverse morphologies suggest that subpopulations are present. We conclude that cell migration from undamaged areas, as well as cell proliferation, is necessary for CNS repair in vitro.
Similar content being viewed by others
References
Chen JS, Levi-Montalcini R (1969) Axonal outgrowth and cell migration in vitro from nervous system of cockroach embryos. Science, New York, 116:631–632
Fujita S, Tsuchihashi Y, Kitamura T (1981) Origin, morphology, and function of the microglia. In: Vidrio EA, Federoff S (eds) Glial and Neuronal Cell Biology. Alan Liss Inc., New York, pp 141–170
Howes EA, McLaughlin BJ, Heslop JP (1974) The autoradiographical association of fast transported material with dense core vesicles in the central nervous system of Anodonta cygnea (L.). Cell Tissue Res 153:545–558
Howes EA, Leech CA (1985) Organ culture of adult cockroach CNS: ultrastructural and physiological characteristics. Tissue Cell 17:141–145
Kuo MT (1981) Preferential damage of active chromatin by bleomycin. Cancer Res 41:2439–2443
Latov N, Nilaver G, Zimmerman EA, Johnson WG, Silvcrman A, Defendini R, Cote L (1979) Fibrillary astrocytes proliferate in response to brain injury. Dev Biol 72:381–384
Ludwin SK (1984) Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature 308:274–275
Nelson JW, Tinoco I Jr (1984) Intercalation of ethidium ion into DNA and RNA oligonucleotides. Biopolymers 23:213–233
Raff MC, Miller RH, Noble M (1983) A glial progenitor cell that develops in vitro into astrocytes or an oligodendrocyte depending on culture medium. Nature 303:390–396
Salzer JE, Bunge RP (1980) Studies of Schwann cell proliferation. I. An analysis of tissue culture proliferation during development, Wallerian degeneration and direct injury. J Cell Biol 84:739–752
Scharrer B (1972) Cytophysiological features of hemocytes in cockroaches. Z Zellforsch 129:301–309
Schofield PK, Treherne JE (1984) Localization of the blood brain barrier of an insect: Electrical model and analysis. J Exp Biol 109:319–331
Smith PJS, Howes EA (1984) Glial toxin effect on protein synthesis in an insect connective. J Cell Sci 70:83–92
Smith PJS, Howes EA (1986) Neural repair in an insect central nervous system: cell kinetics and proliferation after selective glial disruption. Cell Tissue Res 247:129–135
Smith PJS, Leech CA, Treherne JE (1984) Glial repair in an insect central nervous system: Effects of selective glial disruption. J Neurosci 4:2698–2711
Smith PJS, Howes EA, Leech CA, Treherne JE (1986) Haemocyte involvement in the repair of the insect central nervous system after selective glial disruption. Cell Tissue Res 243:367–374
Treherne JE, Harrison JB, Treherne JM, Lane NJ (1984) Glial repair in an insect central nervous system: Effect of surgical lesioning. J Neurosci 4:2689–2697
Treherne JE, Howes EA, Leech CA, Smith PJS (1986) The effects of an anti-mitotic drug, bleomycin, on glial repair in an insect central nervous system. Cell Tissue Res 243:375–384
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Howes, E.A., Smith, P.J.S. & Treherne, J.E. Glial repair in the cultured central nervous system of an insect. Cell Tissue Res. 247, 111–120 (1987). https://doi.org/10.1007/BF00216553
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216553