Summary
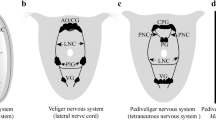
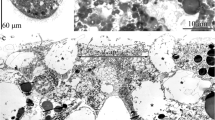
Two pairs of ganglia are found in the propodial region of the veliger of Onchidoris bilamellata: the anterolateral pair is located at the foremost corners of the propodium, and the frontal pair is located beside the propodial midline. Both sets of ganglia are positioned below the epidermis, and they are joined to the cerebral ganglia by large, common connectives. Each ganglion possesses sensory cells, nerve cells and sheath cells, and the frontal pair contains a complement of secretory cells. Externally, the propodial ganglia are manifested as sensory fields. The fields of the anterolateral pair are elliptical in shape, and each appears as a band of cilia bordering an unciliated zone. The region devoid of cilia is composed of ordinary epidermal cells, whereas the ciliated portion is comprised of dendritic endings originating from cells in the ganglion. Dendrites arise from one type of sensory cell and pass through the epidermis in bundles. Each dendrite terminates as a single cilium at the epidermal surface. Sensory fields of the frontal ganglia are key-shaped and oppose one another on the anterior end of the foot. Each field appears as a flat, circular, unciliated region which extends into a ciliated groove that runs dorsally toward the mouth. The groove contains the terminals of secretory cells, ciliated sensory cells, and the cell bodies of nonciliated sensory cells. The nonciliated sensory cells, characterized by a microvillous apex, are the dominant cells in the flattened circular zone. The space between the frontal ganglia and the epidermis is bridged by bundles of processes which are similar to those of the anterolateral ganglia. However, these tracts contain collections of the apical processes of secretory cells, the dendrites of ciliated sensory cells, and the axons of nonciliated sensory cells. Morphological and behavioral evidence indicates that the propodial ganglia serve a chemosensory function during settlement and metamorphosis.
Similar content being viewed by others
References
Agersborg HPK (1922) Some observations on qualitative chemical and physical stimulations in nudibranch molluscs, with special reference to the role of the “rhinophores”. J Exp Zool 36:423–445
Altner H, Prillinger L (1980) Ultrastructure of invertebrate chemoreceptors, thermoreceptors and hydroreceptors, and its functional significance. Int Rev Cytol 67:69–140
Arey LB (1918) The multiple sensory activities of the so-called rhinophore of nudibranchs. Am J Physiol 46:526–532
Arkett SA, Mackie GO, Singla CL (1987) Neuronal control of ciliary locomotion in a gastropod veliger (Calliostoma). Biol Bull 173:513–526
Audesirk G, Audesirk T (1980a) Complex mechanoreceptors in Tritonia diomedea. I. Responsiveness to mechanical and chemical stimuli. J Comp Physiol 141:101–109
Audesirk G, Audesirk T (1980b) Complex mechanoreceptors in Tritonia diomedea. II. Neuronal correlates of a change in behavioral responsiveness. J Comp Physiol 141:111–122
Baloun AJ, Morse DE (1984) Ionic control of metamorphosis in larval Haliotis rufescens (Gastropoda). Biol Bull 167:124–138
Bickell LR (1978) Larval development, metamorphosis, and juvenile feeding of Doridella steinbergae (Lance) (Opisthobranchia: Nudibranchia). MSc Thesis, The University of Alberta, Edmonton, AB, Canada
Bickell LR, Chia FS (1979) Organogencsis and histogenesis in the planktotrophic veliger of Doridella steinbergae (Opisthobranchia: Nudibranchia). Mar Biol 52:291–313
Bickell LR, Kempf SC (1983) Larval and metamorphic morphogenesis in the nudibranch Melibe leonina (Mollusca: Opisthobranchia). Biol Bull 165:119–138
Bonar DB (1976) Molluscan metamorphosis: a study in tissue transformation. Am Zool 16:573–591
Bonar DB (1978a) Ultrastructure of a cephalic sensory organ in larvae of the gastropod Phestilla sibogae (Aoelidacea, Nudibranchia). Tissue Cell 10:153–165
Bonar DB (1978b) Morphogenesis at metamorphosis in opisthobranch molluscs. In: Chia FS, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North Holland, New York, pp 177–196
Bonar DB, Hadfield MG (1974) Metamorphosis of the marine gastropod Phestilla sibogae Bergh (Nudibranchia: Aoelidacea). I. Light and electron microscope analysis of larval and metamorphic stages. J Exp Mar Biol Ecol 16:227–255
Bubel A (1983) Epidermal cells. In: Willows AOD (ed) The Mollusca, vol 7, Reproduction. Academic Press, New York, pp 400–447
Burke RD (1983) The induction of metamorphosis of marine invertebrate larvae: stimulus and response. Can J Zool 16:1701–1719
Chase R, Kroll R (1981) Tentacular function in snail olfactory orientation. J Comp Physiol 143A: 357–362
Chia FS (1978) Perspectives: settlement and metamorphosis of marine invertebrate larvae. In: Chia FS, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North Holland, New York, pp 283–285
Chia FS, Koss R (1982) Fine structure of the larval rhinophores of the nudibranch, Rostanga pulchra, with emphasis on the sensory receptor cells. Cell Tissue Res 225:235–248
Chia FS, Koss R (1984) Fine structure of the cephalic sensory organ in the larva of the nudibranch Rostanga pulchra (Mollusca, Opisthobranchia, Nudibranchia). Zoomorphology 104:131–139
Chia FS, Koss R (1988) Induction of settlement and metamorphosis of the veliger larva of the nudibranch, Onchidoris bilamellata. Int J Invert Reprod (in press)
Chia FS, Atwood DG, Crawford BJ (1975) Comparative morphology of echinoderm sperm and possible phylogenetic implications. Am Zool 15:553–565
Cloney RA (1982) Ascidian larvae and the events of metamorphosis. Am Zool 22:817–826
Crisp M (1971) Structure and abundance of unspecialized external epithelium of Nassarius reticulatus (Gastropoda, Prososbranchia). J Mar Biol Assoc UK 51:865–890
Dorsett DA (1986) Brains to cells: the neuroanatomy of selected gastropod species. In: Willows AOD (ed) The Mollusca, vol 9, Neurobiology and behavior, pt 2. Academic Press, Orlando, pp 101–187
Emery DG, Audesirk TE (1978) Sensory cells in Aplysia. J Neurobiol 9:173–179
Hadfield MG (1978) Metamorphosis of marine molluscan larvae: analysis of stimulus and response. In: Chia FS, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier/North Holland, New York, pp 403–413
Hirata K, Hadfield MG (1986) The role of choline in metamorphic induction of Phestilla (Gastropoda: Nudibranchia). Comp Biochem Physiol 84C: 15–21
Kataoka S (1976) Fine structure of the epidermis of the optic tentacle in a slug, Limax flavus. Tissue Cell 8:33–46
Kohn AJ (1983) Feeding biology of gastropods. In: Saleuddin ASM (ed) The Mollusca, vol 5, Physiology, pt 2. Academic Press, Orlando, pp 1–63
Laverack MS (1974) The structure and function of chemoreceptor cells. In: Grant PT, Mackie AM (eds) Chemoreception in marine organisms. Academic Press, New York, pp 1–48
Mackie GO, Singla CL, Thiriot-Quievreux C (1976) Nervous control of ciliary activity in gastropod larvae. Biol Bull 151:182–199
Matera EM, Davis WJ (1982) Paddle cilia (discocilia) in chemosensilive structures of the gastropod mollusk Pleurobranchia California. Cell Tissue Res 222:25–40
McEuen FS (1985) Reproductive patterns in holothuroids. PhD Thesis, The University of Alberta, Edmonton, AB, Canada
Morse DE, Hooker N, Duncan H, Jensen L (1979) γ-aminobutyric acid, a neurotransmitter, induces planktonic abalone larvae to settle and begin metamorphosis. Science 204:407–410
Morse DE, Duncan H, Hooker N, Baloun A, Young G (1980) GABA induces behavioral and developmental metamorphosis in planktonic molluscan larvae. Fed Proc 39:3237–3241
Phillips CFA (1979) Ultrastructure of sensory cells on the mantle tentacles of the gastropod Notoacmaea scutum. Tissue Cell 11:623–632
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Storch V, Welsch V (1969) Über Bau und Funktion der Nudibranchier-Rhinopheron. Z Zellforsch 97:528–536
Thiriot-Quievreux C (1970) Transformations histologiques lors de la métamorphose chez Cymbulia peroni de Blainville (Mollusca, Opisthobranchia). Z Morphol Tiere 67:106–117
Thiriot-Quievreux C (1977) Veligere planctotrophe du doridien Aegires puctilucens (D'Orbigny) (Mollusca: Nudibranchia: Notodorididae): description et metamorphose. J Exp Mar Biol Ecol 26:177–196
Thompson TE (1958) The natural history, embryology, larval biology, and post-larval development of Adalaria proxima (Alder and Hancock) (Gastropoda, Opisthobranchia). Philos Trans R Soc Lond [Biol] 242:1–57
Thompson TE (1962) Studies on the ontogeny of Tritonia hombergi Cuvier (Gastropoda, Opisthobranchia). Philos Trans R Soc Lond [Biol] 245:171–218
Todd CD (1981) The ecology of nudibranch molluscs. Oceanogr Mar Biol Rev 19:141–234
Trapido-Rosenthal H, Morse DE (1985) L-diamino acids facilitate GABA induction of larval metamorphosis in a gastropod mollusc (Haliotis rufescens). J Comp Physiol 155B:403–414
Willows AOD (1978) Physiology and feeding in Tritonia. I. Behavior and mechanics. Mar Behav Physiol 5:115–135
Wolter H (1967) Beiträge zur Biologie, Histologie und Sinnesphy-siologie (insbesondere Chemorezeption) einiger Nudibranchier (Mollusca, Opisthobranchia) der Nordsee. Z Morphol Okol 60:275–337
Wood RL, Luft JH (1965) The influence of buffer systems on fixation with osmium tetroxide. J Ultrastruct Res 12:22–45
Wright BR (1974a) Sensory structure of the tentacles of the slug Arion ater (Pulmonata, Mollusca). 1. Ultrastructure of the distal epithelium, receptor cells and tentacular ganglion. Cell Tissue Res 151:229–244
Wright BR (1974b) Sensory structure of the tentacles of the slug Arion ater (Pulmonata, Mollusca). 2. Ultrastructure of the free nerve endings in the distal epithelium. Cell Tissue Res 151:245–257
Yool AJ, Grau SM, Hadfield MG, Jensen RA, Markell DA, Morse DE (1986) Excess potassium induces larval metamorphosis in four marine invertebrate species. Biol Bull 170:255–266
Zylstra U (1972) Distribution and ultrastructure of epidermal sensory cells in the fresh water snails Lymnaea stagnalis and Biomphalaria pfeifferi. Netherl J Zool 22:283–289
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chia, FS., Koss, R. The fine structure of the newly discovered propodial ganglia of the veliger larva of the nudibranch Onchidoris bilamellata . Cell Tissue Res. 256, 17–26 (1989). https://doi.org/10.1007/BF00224714
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224714