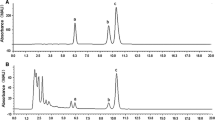
Abstract
The changes in photosynthetic efficiency and photosynthetic pigments during dehydration of the resurrection plantSelaginella lepidophylla (from the Chiuhahuan desert, S.W. Texas, USA) were examined under different light conditions. Changes in the photosynthetic efficiency were deduced from chlorophyll a fluorescence measurements (Fo, Fm, and Fv) and pigment changes were measured by HPLC analysis. A small decrease in Fv/Fm was seen in hydrated stems in high light (650 μmol photons·m−2·s−1) but not in low light (50 μmol photons·m−2·s−1). However, a pronounced decline in Fv/Fm was observed during dehydration in both light treatments, after one to two hours of dehydration. A rise in Fo was observed only after six to ten hours of dehydration. Concomitant with the decrease in photosynthetic efficiency during dehydration a rise in the xanthophyll zeaxanthin was observed, even in low-light treatments. The increase in zeaxanthin can be related to previously observed photoprotective non-photochemical quenching of fluorescence in dehydrating stems ofS. lepidophylla. We hypothesize that under dehydrating conditions even low light levels become excessive and zeaxanthin-related photoprotection is engaged. We speculate that these processes, as well as stem curling and self shading (Eickmeier et al. 1992), serve to minimize photoinhibitory damage toS. lepidophylla during the process of dehydration.
Similar content being viewed by others
Abbreviations
- EPS:
-
epoxidation status
- Fo :
-
initial fluorescence
- Fm :
-
maximal fluorescence
- Fv :
-
variable fluorescence
- PPFD:
-
photosynthetic active photon flux density
- PS:
-
photosystem
- RWC:
-
relative water content
References
Adams WW, Demmig-Adams B, Winter K (1990) Relative contribution of zeaxanthin-related and zeaxanthin-unrelated types of ‘high-energy-state’ quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol 92:302–309
Adams WW, Demmig-Adams B (1992) Operation of the xanthophyll cycle in higher plants in response to diurnal changes in inident sunlight. Planta 186:390–398
Bergtrom G, Schaller M, Eickmeier WG (1982) Ultrastructural and biochemical bases of resurrection in the drought-tolerant vascular plant,Selaginella lepidophylla. J Ultrastr Res 78:269–282
Bewley JD, Krochko JE (1982) Desiccation-tolerance. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. (Encyclopedia in plant physiology, NS, vol 12B). Springer, Berlin Heidelberg New York, pp 325–378
Bilger W, Rinke S, Schreiber U, Lange OL (1989) Inhibition of energy-transfer to photosystem II in lichens by dehydration: different properties of reversibility with green and blue-green phycobionts. J Plant Physiol 134:261–268
Björkman O (1987) Low-temperature chlorophyll fluorescence in leaves and its relationship to photon yield of photosynthesis in photoinhibition. In: Barber J (ed) Photoinhibition. Elsevier, Amsterdam New York Oxford, pp 123–144
Demmig-Adams B (1989) Lichtstress und Lichtschutz bei Pflanzen. Naturwissensch 76:262–267
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim et Biophys Acta 1020:1–24
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171:171–184
Demmig B, Winter K, Krueger A, Czygan F-C (1988) Zeaxanthin and the heat dissipation of excess light energy inNerium oleander exposed to a combination of high light and water stress. Plant Physiol 87:17–24
Demmig-Adams B, Adams WW, Winter K, Meyer A, Schreiber U, Pereira JS, Krueger A, Czygan F-C, Lange OL (1989) Photochemical efficiency of photosystem II, photon yield of O2 evolution, photosynthetic capacity, and carotenoid composition during the midday depression of net CO2 uptake inArbutus unedo growing in Portugal. Planta 177:377–387
Demmig-Adams B, Adams WW (1992a) Photoprotection and other responses of plants of high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Demmig-Adams B, Adams WW (1992b) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15:411–419
Eickmeier WG (1980) Photosynthetic recovery of resurrection spikemosses from different hydration regimes. Oecologia 46:380–385
Eickmeier WG, Lebkuecher JK, Osmond CB (1992) Photosynthetic water oxidation and water stress in Plants. In: Somero GN, Osmond CB, Bolis LS (eds) Water and Life: comparative analysis of water relationships at the orgasmic, cellular, and molecular levels. Springer, Berlin Heidelberg New York, pp 223–239
Eickmeier WG, Casper C, Osmond CB (1993) Chlorophyll fluorescence in the resurrection plantSelaginella lepidophylla and evidence for zeaxanthin-associated photoprotection. Planta 189:30–38
Gaff DF (1980) Protoplasmic tolerance of extreme water stress. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress John Wiley and sons, New York Chichester Brisbane Toronto, pp 207–230
Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a nonendcapped, lightly carbon-loaded C-18 HPLC column. J Chromatogr 543:137–145
Harten JB, Eickmeier WG (1987) Comparative desiccation tolerance of three desert pteridophytes: response to long-term desication. Am Midl Nat 118:337–347
Heifetz PB, Lers AL, Boynton JE, Gillham NW, Osmond CB (1992) Photosynthetic consequences of specific chloroplast gene mutations affecting function and synthesis of the photosystem II D1 protein. In: Murata N (ed) Research in Photosynthesis, vol III. Kluwer Academic Publ., Dordrecht, pp 417–420
Hiller W, Wydrzynski T, Siebert M (1992) Isolation of photosystem II protein components in a non-aqueous medium. In: Murata N (ed) Research in Photosynthesis, vol III. Kluwer Academic Publ., Dordrecht, pp 167–170
Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ (1991) Control of the light-harvesting function of chloroplast membranes by aggregation of the LHC II chlorophyll-protein complex. FEBS 292:1–4
Jones BL, Porter JW (1986) Biosynthesis of carotenes in higher plants. CRC critical Rev Plant Sci 3:295–324
Krause GH (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74:566–574
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kyle DJ (1987) The biochemical basis for photoinhibition of photosystem II. In: Kyle DJ, Osmond CB, Amtzen CJ (eds) Photoinhibition. Elsevier, Amsterdam New York Oxford, pp 197–226
Lebkuecher JG, Eickmeier WG (1991) Reduced photoinhibition with stem curling in the resurrection plantSelaginella lepidophylla. Oecologia 88:597–604
Osmond CB, Ramus J, Franklin LA, Levavasseur G, Henley WJ (1993) Fluorescence quenching analysis of photoprotection and photoinhibitory damage inUlva rotundata. Planta (in press)
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Schwab KB, Schreiber U, Heber U (1989) Response of photosynthesis and respiration of resurrection plants to desiccation and rehydration. Planta 177:217–227
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:311–343
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casper, C., Eickmeier, W.G. & Osmond, C.B. Changes of fluorescence and xanthophyll pigments during dehydration in the resurrection plantSelaginella lepidophylla in low and medium light intensities. Oecologia 94, 528–533 (1993). https://doi.org/10.1007/BF00566968
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00566968