Summary
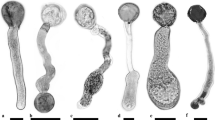
Pollen tubes of Camellia japonica grew toward the cathode upon exposure to an electric field. Reversal of the field direction during the course of tube growth also reversed the direction of tube growth. These observations demonstrate the existence of the electrotropism of pollen tubes. This electro tropic response was confirmed in several kinds of pollen. The direction that the growing tube turned was found to differ with different pollen species, but it did not vary in those of the genus Camellia. The curvature in response to the electrotropic stimulus was influenced by calcium ion concentration as well as by the strength of the applied fields. The optimum condition for each was studied. The degree of tube extension decreased generally in inverse proportion to current density.
Similar content being viewed by others
References
Heslop-Harrison J, Heslop-Harrison Y (1986) Pollen-tube chemotropism: factor or delusion? In: Cresti M, Dallai R (eds) Biology of reproduction and cell motility in plants and animals. University of Siena, Siena Italy, pp 169–174
Hirouchi T, Suda S (1975) Thigmotropism in the growth of pollen tubes of Lilium longiflorum. Plant Cell Physiol 16:337–381
Iwanami Y (1957) Physiological researches of pollen XIII. Growth inhibition of the pollen tube of Camellia japonica. Bot Mag 70:146–149
Marsh G, Beams HW (1945) The orientation of pollen tubes of Vinca in the electric current. J Cell Comp Physiol 25:195–204
Mascarenhas JP (1973) Pollen tube chemotropism. In: Perez-Miravete A (ed) Behaviour of micro-organisms. Plenum Press, London New York, pp 62–69
Mascarenhas JP, Machlis L (1962) The pollen-tube chemotropism factor from Antirrhinum majus: bioassay, extraction, and partial purification. Am J Bot 49:482–489
Mascarenhas JP, Machlis L (1964) Chemotropic response of the pollen of Antirrhium majus to calcium. Plant Physiol 39:70–77
Miki H (1954) A study of tropism of pollen tubes to the pistil. I. Tropism in Lilium. Bot Mag 67:791–792
Miki H (1955) A study of tropism of pollen tubes to pistil. II. Tropism in Camellia sinensis. Bot Mag 68:293–298
Miki-Hiroshige H (1964) Tropism of pollen tubes to the pistils. In: Linskens HF (ed) Pollen physiology and fertilization. North-Holland Publ, Amsterdam, pp 152–158
Nakamura N (1978) Physiological studies on the pollen growth of Camellia japonica L. in vitro. J Yokohama City Univ Biol Ser 5:1–100
Nakamura N, Mochizuki K, Suzuki H (1988) Growth promoters of the pollen tube of Camellia japonica. II. Stimulated growth on Good's buffers and cooperation of calcium. Jpn J Palynol 34:59–62
Picton JM, Steer MW (1983) Evidence for the role of Ca2+ ions in tip extension in pollen tubes. Protoplasma 115:11–17
Picton JM, Steer MW (1985) The effects of ruthenium red, lanthanum, fluorescein isothiocyanate and trifluorperazine on vesicle transport, vesicle fusion and tip extension in pollen tubes. Planta 163:20–26
Reiss H-D, Herth W, Schnepf E (1983) The tip-to-base calcium gradient in pollen tubes of Lilium longiflorum measured by proton-induced x-ray emission (PIXE). Protoplasma 115:153–159
Reiss H-D, Herth W, Nobiling R (1985) Development of membrane- and calcium-gradients during pollen germination of Lilium longiflorum. Planta 163:84–90
Rosen WG (1961) Studies on pollen-tube chemotropism. Am J Bot 48:889–895
Rosen WG (1962) Cellular chemotropism and chemotaxis. Q Rev Biol 37:242–259
Tsao T-H (1949) A study of chemotropism of pollen tubes in vitro. Plant Physiol 24:494–504
Tsuchiya H (1988) Electrotropism of Camellia japonica pollen growing in a sugar-agar medium. Jpn J Palynol 34:69–70
Wang C, Rathore KS, Robinson KR (1989) The response of pollen to applied electrical fields. Dev Biol 136:405–410
Weisenseel MH, Jaffe LF (1976) The major growth current through lily pollen tubes enters as K+ and leaves as H+. Planta 133:1 -7
Weisenseel MH, Nuccitelli R, Jaffe LF (1975) Large electrical currents traverse growing pollen tubes. J Cell Biol 66:556–567
Welk M, Sr, Millington WF, Rosen WG (1965) Chemotropic activity and the pathway of the pollen tube in lily. Am J Bot 52:774–781
Wulff HD (1935) Galvanotropismus bei Pollenschläuchen. Planta 24:602–608
Zeijlemaker FCJ (1956) Growth of pollen tubes in vitro and their reaction on potential differences. Acta Bot Neerl 5:179–186
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, N., Fukushima, A., Iwayama, H. et al. Electrotropism of pollen tubes of camellia and other plants. Sexual Plant Reprod 4, 138–143 (1991). https://doi.org/10.1007/BF00196501
Issue Date:
DOI: https://doi.org/10.1007/BF00196501