Abstract
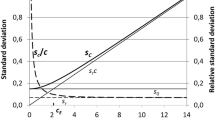
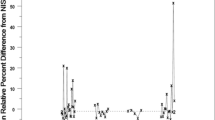
Repeated subsampling or a hierarchical design of experiments combined with an analysis of variance (ANOVA) is demonstrated to be a useful tool in the determination of uncertainty components in amount-of-substance measurements. With the reference material of human serum as investigated here for total cholesterol, besides several in-laboratory sources of uncertainty, a vial-to-vial effect which can be regarded as an off-laboratory source was found to be significant. This knowledge might be essential when the material is used for calibration and for the self-assessment of a laboratory.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 October 1997 · Accepted: 26 November 1997
Rights and permissions
About this article
Cite this article
Henrion, A. In- and off-laboratory sources of uncertainty in the use of a serum standard reference material as a means of accuracy control in cholesterol determination. Accred Qual Assur 3, 127–130 (1998). https://doi.org/10.1007/s007690050204
Issue Date:
DOI: https://doi.org/10.1007/s007690050204