Abstract
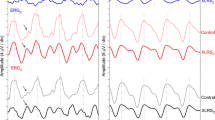
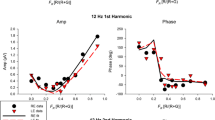
We recorded the electro-oculogram from 27 normal subjects by means of green and near-ultraviolet (UVA) stimulation. After a 40-minute dark-adaptation period, baseline responses were recorded. In response to the green stimulus, the electro-oculogram increased from this level by an average (± standard error of the mean) of 49.5% ±4.0%. Although the predicted scotopic effectiveness of the ultraviolet stimulus was more than 3 log units below that of the green stimulus, the near-ultraviolet-induced electro-oculogram increased to an average of 21.9% ±3.0% above baseline. This response cannot be due to lens fluorescence to the near-ultraviolet stimulus, since two aphakic subjects had electro-oculographic responses of 32% and 76% above baseline to near-ultraviolet stimuli. Neither the green nor the ultraviolet electro-oculogram changed significantly with age. These large responses to near-ultraviolet stimulation demonstrate the need for standardizing light sources for electro-oculographic testing because the degree of near-ultraviolet irradiance varies considerably according to their design characteristics.
Similar content being viewed by others
References
Arden GB, Barrada A, Kelsey JH. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol 1962; 46: 449–67.
Steinberg RH, Linsenmeier RA, Griff ER. Retinal pigment epithelial cell contributions to the electroretinogram and electrooculogram. In: Osborne N, Chader G, eds. Progress in retinal research. New York: Pergamon Press, 1985; 4: 33–66.
Fishman GA. The electro-oculogram in retinal disorders. In: Fishman GA, Sokol S, eds. Electrophysiological testing in disorders of the retina, optic nerve and visual pathway. San Francisco: American Academy of Ophthalmology, 1990: 91–103.
Jones RM, Stevens TS, Gould S. Normal EOG values of young subjects. Doc Ophthalmol Proc Ser 1977; 13: 93–7.
Arden GB, Kelsey JH. Some observations on the relationship between the standing potential of the human eye and the bleaching and regeneration of visual purple. J Physiol 1962; 161: 205–26.
Elenius V, Lehtonen J. Spectral sensitivity of the standing potential of the human eye. Acta Ophthalmol 1962; 40: 559–66.
Friedman AH. The importance of defining light parameters in chronobiological studies. Chronobiol Int 1984; 1: 229–30.
Taumer R, Hennig J, Pernice D. The ocular dipole. A damped oscillator stimulated by the speed of change in illumination. Vision Res 1974; 14: 637–45.
Reimslag F, Lunel HV, Spekreijse H. lectrooculogram. A refinement of the method, Doc Ophthalmol 1990; 73: 369–75.
Dawson WM, Maida TM. Reduced variability in the electrooculogram. Am J Ophthalmol 1984; 97: 395–6.
Maggiano JM, Marchese AL, Friedman AH. The electrooculogram in response to near UV and near IR [ARVO Abstracts]. 1975: 55.
Wyszecki G, Stiles WS. Color science. Concepts and methods, quantitative data and formulae, 2nd ed. New York: John Wiley & Sons, 1989.
Sliney DH, Freasier BC. Evaluation of optical radiation hazards. Appl Opt 1973; 12: 1–22.
Lerman S. Lens proteins and fluorescence. Isr J Med Sci 1972; 8: 1583–9.
Zigman S, Groff J, Yulo T, Griess G. Light extinction and protein in lens. Exp Eye Res 1976; 23: 555–67.
Geeraets WJ, Berry ER. Ocular spectral characteristics as related to hazards from lasers and other light sources. Am J Ophthalmol 1968; 66: 15–20.
Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol 1962; 1: 776–83.
Williams TT, White CW, Stark WS. Ultraviolet visibility after cataract extraction. Invest Ophthalmol Vis Sci 1983; 24 (suppl): 185.
Tan KEWP. Vision in the ultraviolet. Doctoral thesis. Utrecht, 1972.
Wald G, Brown PK, Smith PH. Iodopsin. J Gen Physiol 1954; 38: 623–81.
Sliney DH, Rachlin JA, Marshall WJ. Evaluation of hyperbilirubinemia lamps. Radiation Protection Spectral Study No. 42–025–74/75. Aberdeen Proving Ground, Md: US Army Environmental Health Agency, 1974: 1–13.
International Standardization Committee, International Society for the Clinical Electrophysiology of Vision. Standard for clinical electroretinography. Arch Ophthalmol 1989; 107: 816–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marchese, A.L., Maggiano, J.M. & Friedman, A.H. Comparison of the human electro-oculographic response to green and near-ultraviolet stimuli. Doc Ophthalmol 79, 117–124 (1992). https://doi.org/10.1007/BF00156571
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00156571