Abstract
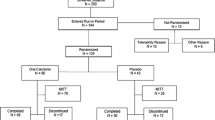
Alendronate sodium (ALN) is a potent amino bisphosphonate which specifically inhibits osteoclastic bone resorption and has been found to reverse bone loss in several animal models. To determine if daily oral ALN treatment could prevent or reverse bone loss in osteoporotic postmenopausal women, and to compare ALN to intranasal salmon calcitonin (CT), a 2-year, double-masked, randomized, placebo-controlled study was initiated at 9 clinical centers in Italy. Two hundred and eighty six postmenopausal women (age 48–76) with spinal bone mineral density (BMD) ≥2 SD below adult mean peak, with or without vertebral crush fractures, were randomized to one of four treatment arms: ALN 10 mg daily, ALN 20 mg daily or matching placebo (these groups all double-masked), or CT 100 IU daily (open label) for 2 years. All patients received supplemental calcium (as carbonate) 500 mg daily. Bone mass was measured by dual-energy X-ray absorptiometry of the PA lumbar spine (LS) and proximal femur (femoral neck and trochanter) at 6-month intervals. Subject safety was measured through sequential clinical and laboratory evaluation. A planned 1-year interim analysis of this ongoing study was performed cetrally in a manner that maintains the double-mask for all subjects receiving oral study drug. Relative to PBO, ALN at either 10 mg or 20 mg daily increased LS BMD by 4.7% and 6.1%, respectively; each increased femoral neck BMD by 3.1% and increased trochanter BMD by 3.3% and 3.8% respectively. In contrast, CT failed to significantly increase BMD of either the spine, femoral neck or trochanter, either relative to baseline or to PBO. ALN decreased biochemical markers or bone turnover, whereas both PBO and CT were ineffective. No serious adverse experiences attributable to the use of alendronate were detected. In summary, daily oral ALN for one year appears to be effective in decreasing bone turnover and increasing bone mass at the spine and the hip. In contrast, daily CT 100 IU had no significant effects either to reduce bone turnover or to increase bone mass at either site. In conclusion, ALN effectively increased bone mass in osteoporotic menopausal women, and was associated with an excellent safety profile.
Similar content being viewed by others
References
Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med 1986;314:1676–86.
Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biochemical consequences. Calcif Tissue Int 1984;36:123–8.
Riggs BL. Toward optimal therapy of established osteoporosis: evidence that antiresorptive and formation stimulating regimens reduce vertebral fractures by independent mechanisms. In: Proceedings of the fourth international symposium on osteoporosis, Hong Kong, 1993:S30.
Parfitt AM. Pathophysiology of bone fragility. In: Proceedings of the fourth international symposium on osteoporosis. Hong Kong, 1993:S43.
Lindsay R, Tohme JF. Estrogen treatment of patients with established osteoporosis. Obstet Gynecol 1990;76:290–5.
Ryan PJ, Harrison R, Blake GM, Fogelman I. Compliance with hormone replacement therapy (HRT) after screening for postmenopausal osteoporosis. Br J Obstet Gynaecol 1992;99:325–8.
Riggs BL, Melton LJ. The prevention and treatment of osteoporosis. N Engl J Med 1992;327:620–7.
Clissold SP, Fitton A, Chrisp A. Intranasal salmon calcitonin: a review of its pharmacological properties and potential utility in metabolism bone disorders associated with aging. Drugs Aging 1991;1:405–23.
Sato M, Grasser W, Endo N, et al. Bisphosphonate action: alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 1991;88:2095–105.
Seedor JG, Quartuccio H, Thompson DD. The bisphosphonate alendronate inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 1991;6:339–46.
Thompson DD, Seedor JG, Quartuccio H, Solomon H, Fioravanti C, Davidson J, et al. The bisphosphonate, alendronate, prevents bone loss in ovariectomized baboons. J Bone Miner Res 1992;7:951–60.
Seedor JG, Balena R, Masarachia P, Rodan GA. Comparison of the therapeutic potencies of two bisphosphonates. Bone 1992;13:S116.
Toolan BC, Shea M, Myers ER, et al. The effect of long term alendronate on vertebral strength in ovariectomized baboons and rats. J Bone Miner Res 1991;6(Suppl 1):S248.
Toolan BC, Shea M, Myers ER, Borchers RE, Seedor JG, Quartuccio H, et al. Effects of 4-amino-1-hydroxybutylidene bisphosphonate on bone biomechanics in rats. J Bone Miner Res 1992;7:1399–406.
Black DM, Reiss TR, Karpf DB, Cummings SR. Fracture intervention trial: design and interim recruitment results. Osteoporosis Int 1993;3 (Suppl 3):S29–39.
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. Lancet 1993;341:72–4.
Cooper C, Wickham C, Walsh K. Appendicular skeletal status and hip fracture in the elderly: 14-year prospective data. Bone 1991;12:361–4.
Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988;81:1804–9.
Ross PD, Davis JW, Epstein RS, Wasnich RD. Preexisting fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991;114:919–23.
Stevenson JC, Cust MP, Gangar KF, Hillard TC, Lees B, Whitehead MI. Effects of transdermal versus oral hormone replacement therapy on bone density in spine and proximal femur in postmenopausal women. Lancet 1990;335:265–9.
Schenk R, Eggli P, Fleisch H, Rosini S. Quantitative morphometric evaluation of the inhibitory activity of new aminobisphosphonates on bone resorption in the rat. Calcif Tissue Int 1986;38:342–9.
Boyce BF, Smith R, Fogelman I, Johnston E, Ralston S, Boyle IT. Focal osteomalacia due to low-dose diphosphonate therapy in Paget's disease. Lancet 1984;11:821–4.
Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 1990;323:73–9.
Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent cyclical etidronate on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 1990:5:183–92.
Frost HM. Treatment of osteoporosis by manipulation of coherent bone cell populations. Clin Orthop 1979;143:227–44.
Valkema R, Vismans FJFE, Papapoulos SE, Powels EKJ, Bijvoet OLM. Maintained improvement in calcium balance and bone mineral content in patients with osteoporosis treated with the bisphosphonate APD. Bone Miner 1989;5:183–92.
Santora AC, Bell NH, Chesnut CH, Ensrud K, Genant HK, Grimm R, et al. Oral alendronate treatment of bone loss in postmenopausal osteopenic women. [abstract] J Bone Miner Res 1993:8(Suppl 1):S131.
McClung MR, Yates AJ. Alendronate prevents or reverses bone loss at the spine and hip in recently menopausal women. [abstract] J Bone Miner Res 1993;8(Suppl 1):S141.
Thamsborg G, Storm TL, Sykulski R, Brinch E, Nielsen HK, Sørensen OH. Effect of different doses of nasal salmon calcitonin on bone mass. Calcif Tissue Int 1991;48:302–7.
Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ 1992;305:556–61.
Overgaard K, Riis BJ, Christiansen C, Pødenphant J, Johansen JS. Nasal calcitonin for treatment of established osteoporosis. Clin Endocrinol 1989;30:435–42.
Overgaard K, Hansen MS, Heiss Nielsen VA, Riis BJ, Christiansen C. Discontinuous calcitonin treatment of established osteoporosis: effects of withdrawal of treatment. Am J Med 1990;89:1–6.
Meunier PJ, Gozzo I, Chaumet-Riffaud PD, Delmas PD, Guignard M, Chapuy MC, Duboeuf F. Dose-effect on bone density and parathyroid function of intranasal salmon calcitonin when administered without calcium in postmenopausal women. [abstract]. J Bone Miner Res 1992;7(Suppl 1):abst.950.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adami, S., Baroni, M.C., Broggini, M. et al. Treatment of postmenopausal osteoporosis with continuous daily oral alendronate in comparison with either placebo or intranasal salmon calcitonin. Osteoporosis Int 3 (Suppl 3), 21–27 (1993). https://doi.org/10.1007/BF01623004
Issue Date:
DOI: https://doi.org/10.1007/BF01623004